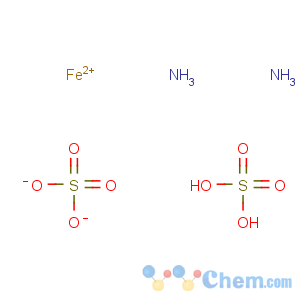
Title: Ammonium Ferrous Sulfate
CAS Registry Number: 10045-89-3
Synonyms: Ferrous ammonium sulfate; Mohr's salt
Molecular Formula: FeH8N2O8S2
Molecular Weight: 284.05
Percent Composition: Fe 19.66%, H 2.84%, N 9.86%, O 45.06%, S 22.58%
Line Formula: (NH4)2Fe(SO4)2
Literature References: Manuf from Fe, H2SO4 and NH3: Demmerle
et al., Ind. Eng. Chem. 42, 9 (1950); from pickling waste: Brundin,
US 2694657 (1954 to Ekstrand and Tholand). Toxicity study: H. F. Smyth
et al., Am. Ind. Hyg. Assoc. J. 30, 470 (1969).
Derivative Type: Hexahydrate
Properties: Pale blue-green crystals or crystalline powder. Slowly oxidizes and effloresces in air. d420 1.86. Soluble in water. Practically insol in alcohol.
Keep well closed and protected from light. LD50 orally in rats: 3.25 g/kg (Smyth).
Density: d420 1.86
Toxicity data: LD50 orally in rats: 3.25 g/kg (Smyth)
Use: In photography; as analytical standard; as polymerization catalyst; in dosimeters.