Title: Glyoxal
CAS Registry Number: 107-22-2
CAS Name: Ethanedial
Synonyms: biformyl; diformyl; oxalaldehyde
Molecular Formula: C2H2O2
Molecular Weight: 58.04
Percent Composition: C 41.39%, H 3.47%, O 55.13%
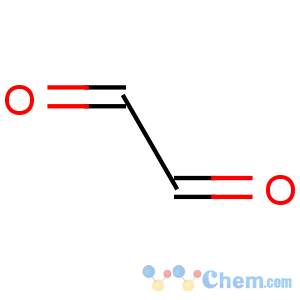
Line Formula: OHCCHO
Literature References: Prepd by the oxidation of acetaldehyde by nitric or selenious acid: Lubawin,
Ber. 8, 768 (1875); Wyss,
Ber. 10, 1366 (1877); K?lln,
Ann. 416, 230 (1918); Riley
et al., J. Chem. Soc. 1932, 1881; Ronzio, Waugh,
Org. Synth. coll. vol. III, 438 (1955); by hydrolysis of dichlorodioxane: Butler, Cretcher,
J. Am. Chem. Soc. 54, 2988 (1932). Review of commercial development: J. F. Bohmfalk
et al., Ind. Eng. Chem. 43, 786 (1951). Toxicity study: H. F. Smyth
et al., J. Ind. Hyg. Toxicol. 23, 259 (1941).
Review: A. B. Boese
et al. in
Glycols, G. O. Curme, F. Johnston, Eds. (Reinhold, New York, 1952) pp 125-128.
Properties: Yellow prisms or irregular pieces turning white on cooling. d20 1.14. Opaque at 10°, mp 15°. bp776 51°. The vapors are green and burn with a purple flame.
Explosive: Mixtures with air may explode!
nD20.5 1.3826. Sol in anhydr solvents. pH of a 40% aq soln: 2.1-2.7; d420 1.27. Polymerizes quickly on standing, on contact with water (violent reaction), or when dissolved in solvents contg water. The anhydr polymer changes to the monomer on heating. Solns of the monomer are obtained on heating the polymer with anethole, phenetole, safrole, methyl nonyl ketone, or benzaldehyde. LD50 in rats, guinea pigs (mg/kg): 2020, 760 orally (Smyth).
Melting point: mp 15°
Boiling point: bp776 51°
Index of refraction: nD20.5 1.3826
Density: d20 1.14; d420 1.27
Toxicity data: LD50 in rats, guinea pigs (mg/kg): 2020, 760 orally (Smyth)
Derivative Type: Dihydrate
Molecular Formula: (OHCCHO)3.2H2O
Molecular Weight: 210.14
Percent Composition: O 60.91%, H 4.80%, C 34.29%
Properties: Crystalline powder, nonhygroscopic. More sol in hot water than in cold water. Commercially available in anhydr form as crystalline dihydrate, or as a 40% aq soln which may contain polymerization inhibitors.
CAUTION: Moderately irritating to skin, mucous membranes.
Use: In textiles, organic synthesis, glues, biocides.