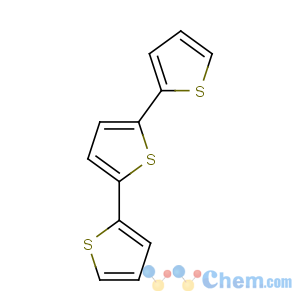
Title: a-Terthienyl
CAS Registry Number: 1081-34-1
CAS Name: 2,2¢:5¢2¢¢-Terthiophene
Synonyms: 5-(2-thienyl)-2,2¢-bithiophene
Molecular Formula: C12H8S3
Molecular Weight: 248.39
Percent Composition: C 58.03%, H 3.25%, S 38.73%
Literature References: Biocidal constituent of various species of marigolds. Isoln from
Tagetes erecta L.,
Compositae: L. Zechmeister, J. W. Sease,
J. Am. Chem. Soc. 69, 273 (1947). Isoln and distribution in
Tageteae: K. R. Downum, G. H. N. Towers,
J. Nat. Prod. 46, 98 (1983). Synthesis: W. Steinkopf
et al., Ann. 546, 180 (1941); H. J. Kooreman, H. Wynberg,
Rec. Trav. Chim. 86, 37 (1967); J.-P. Beny
et al., J. Org. Chem. 47, 2201 (1982). Biological activity as nematocide: J. H. Uhlenbroek, J. D. Bijloo,
Rec. Trav. Chim. 77, 1004 (1958); J. D. Bijloo
et al., DE 1075891;
eidem, US 3050442 (1960, 1962, both to North American Philips); J. Bakker
et al., J. Biol. Chem. 254, 1841 (1979); as herbicide: J. Harvey, Jr.,
US 3086854 (1963 to Du Pont); G. Campbell
et al., J. Chem. Ecol. 8, 961 (1982); as antimicrobial: J. R. Kagan
et al., Photochem. Photobiol. 31, 465 (1980); T. Arnason
et al., ibid. 33, 821 (1981); F. DiCosmo
et al., Pestic. Sci. 13, 589 (1982).
Properties: Yellow-orange plates from methanol, mp 93-94°. uv max (methanol): 254, 350 nm (e 7100, 21300). Sol in carbon bisulfide, ether, benzene, acetone, petr ether; slightly sol in methanol, ethanol. Insol in water.
Melting point: mp 93-94°
Absorption maximum: uv max (methanol): 254, 350 nm (e 7100, 21300)