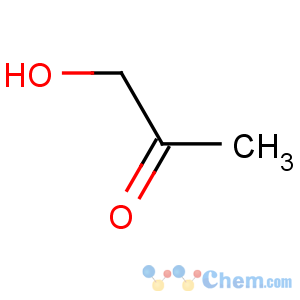
Title: Acetol
CAS Registry Number: 116-09-6
CAS Name: 1-Hydroxy-2-propanone
Synonyms: hydroxyacetone; acetone alcohol; acetylcarbinol; acetylmethanol; 2-oxopropanol
Molecular Formula: C3H6O2
Molecular Weight: 74.08
Percent Composition: C 48.64%, H 8.16%, O 43.19%
Line Formula: H3CCOCH2OH
Literature References: Widely distributed in nature. Produced in animals as an intermediate in the intrahepatic metabolism of acetone. Also formed by the action of aldose reductase on methylglyoxal,
q.v. and accumulates in uncontrolled diabetes. Prepn: W. H. Perkin, Jr.,
J. Chem. Soc. 59, 786 (1891); T. Matsumoto
et al., J. Org. Chem. 50, 603 (1985). Isoln from coffee extract: M. Stoll
et al., Helv. Chim. Acta 50, 628 (1967). HPLC determn in serum: J. P. Casazza, J. L. Fu,
Anal. Biochem. 148, 344 (1985). Metabolism and potential role in diabetic complications: D. L. Vander Jagt
et al., J. Biol. Chem. 267, 4364 (1992). Use in peptide synthesis: B. Kundu,
Tetrahedron Lett. 33, 3193 (1992).
Properties: Colorless oil with peculiar odor. bp20 50°; bp200 105-106°; bp760 147° (dec).
nD20 1.4235. d420 1.0872. Misc with water.
Boiling point: bp20 50°; bp200 105-106°; bp760 147° (dec)
Index of refraction: nD20 1.4235
Density: d420 1.0872
Use: Reagent in organic synthesis; protecting group for the synthesis of peptides.