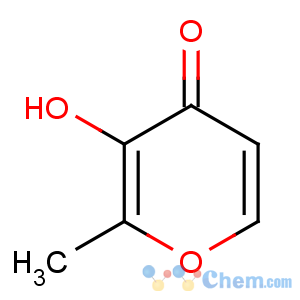
Title: Maltol
CAS Registry Number: 118-71-8
CAS Name: 3-Hydroxy-2-methyl-4
H-pyran-4-one
Synonyms: 3-hydroxy-2-methyl-4-pyrone; 3-hydroxy-2-methyl-g-pyrone; larixinic acid; palatone; veltol
Molecular Formula: C6H6O3
Molecular Weight: 126.11
Percent Composition: C 57.14%, H 4.80%, O 38.06%
Literature References: Found in the bark of young larch trees (
Larix decidua Mill.), in pine needles
(Abies alba Mill.,
Pinaceae), in chicory, in wood tars and oils, in roasted malt. Isoln from these sources and structure: Kiliani, Bazlen,
Ber. 27, 3115 (1894); Feuerstein,
Ber. 34, 1804 (1901); Erdmann, Schaefer,
Ber. 43, 2398 (1910); Reichstein, Beitter,
Ber. 63, 824 (1930),
cf. Peratoner, Tamburello,
Chem. Zentralbl. 76, II 680 (1905). Also obtained by alkaline hydrolysis of streptomycin salts: Schenck, Spielman,
J. Am. Chem. Soc. 67, 2276 (1945). Synthesis: Spielman, Freifelder,
ibid. 69, 2908 (1947); Chawla, McGonigal,
J. Org. Chem. 39, 3281 (1974). Novel high-yield synthesis: T. M. Brennan
et al., Tetrahedron Lett. 1978, 331; P. D. Weeks
et al., J. Org. Chem. 45, 1109 (1980). History and comparison with isomaltol: Hodge, Nelson,
Cereal Chem. 38, 207 (1961).
Properties: Monoclinic prisms from chloroform, orthorhombic bipyramidal crystals + monoclinic prisms from 50% alcohol, mp 161-162°. Fragrant, caramel-like odor. Begins to sublime at 93°. Volatile with steam. uv max (0.1
N HCl): 274 nm (Em 8400); (0.1
N NaOH): 317 nm (Em 7300). pH of 0.5% aq soln 5.3. One gram dissolves in 85 ml water. Freely sol in hot water, chloroform; sol in alcohol; sparingly sol in benzene, ether, petr ether; sol in aq alkali hydroxides giving yellow solns.
Melting point: mp 161-162°
Absorption maximum: uv max (0.1
N HCl): 274 nm (Em 8400); (0.1
N NaOH): 317 nm (Em 7300)
Use: Flavoring agent, to impart "freshly baked" odor and flavor to bread and cakes.