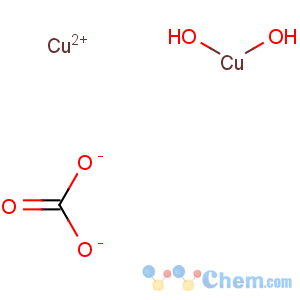
Title: Cupric Carbonate, Basic
CAS Registry Number: 12069-69-1
CAS Name: Copper carbonate hydroxide
Synonyms: cupric subcarbonate; Bremen blue; Bremen green
Molecular Formula: CH2Cu2O5
Molecular Weight: 221.12
Percent Composition: C 5.43%, H 0.91%, Cu 57.48%, O 36.18%
Line Formula: CuCO3.Cu(OH)2
Literature References: Occurs in nature as the mineral
malachite (dark green monoclinic crystals). Prepd by adding CuSO4 soln to Na2CO3 soln: Winter
et al., in
Kirk-Othmer Encyclopedia of Chemical Technology vol. 6 (Interscience, New York, 2nd ed., 1965) p 270. The commercial product usually contains 50-55% Cu.
Properties: Green to blue amorphous powder or dark-green monoclinic crystals. Practically insol in water, alcohol; sol in dil acids, ammonia.
NOTE: Another form of basic copper carbonate, 2CuCO3.Cu(OH)2, occurs in nature as the mineral
azurite or
chessylite.
See also Burgundy Mixture.
Use: As seed treatment fungicide; in pyrotechnics; as paint and varnish pigment; in animal and poultry feeds; in sweetening of petrol sour crude stock; in manuf of other Cu salts.
Therap-Cat-Vet: Nutritional factor. Used in copper deficiency in ruminants.