Title: Vanillin
CAS Registry Number: 121-33-5
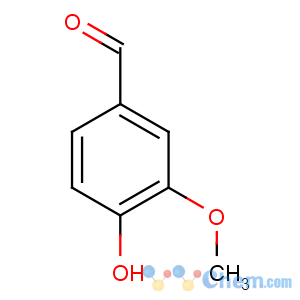
CAS Name: 4-Hydroxy-3-methoxybenzaldehyde
Synonyms: methylprotocatechuic aldehyde; vanillic aldehyde
Trademarks: Rhonavil Extra Pure (Rh>e-Poulenc)
Molecular Formula: C8H8O3
Molecular Weight: 152.15
Percent Composition: C 63.15%, H 5.30%, O 31.55%
Literature References: Occurs naturally in a wide variety of foods and plants such as orchids; major commercial source of natural vanillin is from vanilla bean extract. Synthetically produced in-bulk from lignin-based byproducts of paper processes or from guaicol. Known prior to literature identification, first reported in the literature by Bucholtz in 1816 but not isolated until 1858. Isoln from vanilla beans: M. Gobley,
Jahresber. Fortschr. Chem. Verw. Theile Anderer Wiss. 1858, 534. LC determn: S. Kahan, D. A. Krueger,
J. AOAC Int. 80, 564 (1997). Acute toxicity: P. M. Jenner
et al., Food Cosmet. Toxicol. 2, 327 (1964). Brief review of toxicology: D. L. J. Opdyke,
ibid. 15, 633-638 (1977). Review of NMR studies: G. J. Martin,
Ind. Chem. Libr. 8, 506-527 (1996). Review of synthetic prepn: M. B. Hocking,
J. Chem. Educ. 74, 1055-1059 (1997). Reviews: G. S. Clark,
Perfum. Flavor. 15, 45-54 (1990); L. J. Esposito
et al. in
Kirk-Othmer Encyclopedia of Chemical Technology vol. 24 (Wiley-Interscience, New York, 4th ed., 1997) pp 812-825.
Properties: White or off-white nonhygroscopic crystalline powder. Pleasant aromatic vanilla odor and taste. Affected by light. d 1.056; bulk density ~0.6. mp 81-83°. bp101.3 kPa 285°; bp10 mm Hg 154°. Solubility in water >2%; in ethanol is 1:2 vanillin:ethanol. Freely sol in chloroform, ether, in solns of fixed alkali hydroxides; sol in glycerin and hot water. Solns are acid to litmus. LD50 orally in rats, guinea pigs: 1580, 1400 mg/kg (Jenner).
Melting point: mp 81-83°
Boiling point: bp101.3 kPa 285°; bp10 mm Hg 154°
Density: d 1.056; bulk density ~0.6
Toxicity data: LD50 orally in rats, guinea pigs: 1580, 1400 mg/kg (Jenner)
Use: Pharmaceutic aid (flavor). As a flavoring agent in confectionery, beverages, foods and animal feeds. Fragance and flavor in cosmetics. Reagent for synthesis.