Title: Sodium Bismuthate(V)
CAS Registry Number: 12232-99-4
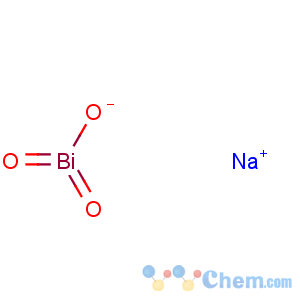
Molecular Formula: BiNaO3
Molecular Weight: 279.97
Percent Composition: Bi 74.64%, Na 8.21%, O 17.14%
Line Formula: NaBiO3
Literature References: The bismuthate of commerce contains about 85% NaBiO3; the balance is chiefly water and Bi2O3.
Properties: Yellow to yellowish-brown, somewhat hygroscopic. Slowly dec on keeping; decompn accelerated by moisture and higher temp. Insol in cold, dec by hot water forming Bi2O3, NaOH, and liberating oxygen; dec by acids; with HCl chlorine is formed; with oxy-acids oxygen is liberated. LD100 orally in rats: 720 mg/kg, Hanzlik
et al., J. Pharmacol. Exp. Ther. 62, 372 (1938).
Toxicity data: LD100 orally in rats: 720 mg/kg, Hanzlik
et al., J. Pharmacol. Exp. Ther. 62, 372 (1938)
Use: For the determination of manganese in iron and steel, etc., the manganese being oxidized by it in hot HNO3 or H2SO4 soln to permanganate.