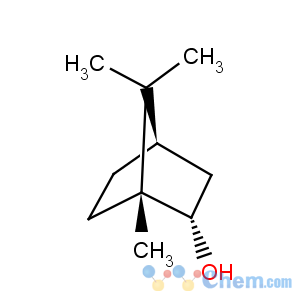
Title: Isoborneol
CAS Registry Number: 124-76-5
CAS Name: exo-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol
Synonyms: exo-2-bornanol;
exo-2-camphanol
Molecular Formula: C10H18O
Molecular Weight: 154.25
Percent Composition: C 77.87%, H 11.76%, O 10.37%
Literature References: Prepn of
dl-form: Pickard, Littlebury,
J. Chem. Soc. 91, 1973 (1907); Truett, Moulton,
J. Am. Chem. Soc. 73, 5913 (1951); Ziegler,
GB 803178 (1958). Prepn of the
l-form by reduction of
d-camphor with lithium aluminum hydride: Trevoy, Brown,
J. Am. Chem. Soc. 71, 1675 (1949). Separation of isoborneol from its
endo-isomer, borneol, via the
p-nitrobenzoate deriv: Truett, Moulton,
loc. cit. Resolution of the
dl-form: Pickard, Littlebury,
loc. cit.; Kenyon, Priston,
ibid. 127, 1472 (1925). Configuration (isoborneol =
exo-form; borneol =
endo-form): Toivonen
et al., Acta Chem. Scand. 3, 991 (1949).
Review: J. L. Simonsen,
The Terpenes vol. II (University Press, Cambridge, 2nd ed., 1949) pp 365-367; A. R. Pinder,
The Chemistry of the Terpenes (Chapman & Hall, London, 1960) pp 22-24, 101, 103, 105-107, 111.
Derivative Type: dl-Form
Properties: Crystals from petr ether. Sublimes on heating, mp 212° (in a sealed tube). Practically insol in water. Readily sol in alcohol, ether, chloroform.
Melting point: mp 212° (in a sealed tube)
Derivative Type: d-Form
Properties: Crystals from petr ether, mp 214°. Approx [a]D +34.3° in alc soln: Picard, Littlebury,
loc. cit.
Melting point: mp 214°
Optical Rotation: [a]D +34.3° in alc soln: Picard, Littlebury,
loc. cit
Derivative Type: l-Form
Properties: Crystals from petr ether, mp 214°. Approx [a]D -34.3° in alc soln.
Melting point: mp 214°
Optical Rotation: Approx [a]D -34.3° in alc soln