Title: Antimony Trioxide
CAS Registry Number: 1309-64-4
Synonyms: Diantimony trioxide; flowers of antimony; exitelite; senarmontite; valentinite; weisspiessglanz
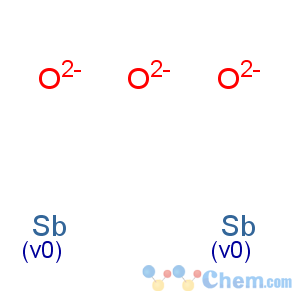
Molecular Formula: O3Sb2
Molecular Weight: 291.52
Percent Composition: O 16.46%, Sb 83.53%
Line Formula: Sb2O3
Literature References: Laboratory prepn from SbCl3 and water: Schenk in
Handbook of Preparative Inorganic Chemistry vol. 1, G. Brauer, Ed. (Academic Press, New York, 2nd ed., 1963) p 615. Obtained from antimony ore minerals by a volatilization (roasting) process: L. D. Freedman in
Kirk-Othmer Encyclopedia of Chemical Technology vol. 3 (Wiley-Interscience, New York, 3rd ed., 1978) pp 107-108. Toxicity study: H. F. Smyth
et al., J. Ind. Hyg. Toxicol. 30, 63 (1948).
Properties: Crystals, polymorphic. mp 655°. bp 1425°; bp210 870°. Sublimes in high vacuum at 400°. Exists in the vapor phase as Sb4O6. Heat capacity at 21° (294.4 K): 24.11 cal/g-atom/°C, Anderson,
J. Am. Chem. Soc. 52, 2712 (1930). Heat of vaporization: 17.82 kcal/mol. Slightly sol in water, dilute H2SO4, or dilute HNO3. Soly in dil HCl (0.1 moles HCl/kg H2O): ~1 ′ 10-4 g-atoms Sb/kg H2O, Gayer, Garrett,
ibid. 74, 2353 (1952). Soly increases with increasing HCl concn: Lea, Wood,
J. Chem. Soc. 125, 137 (1924). Sol in solns of alkali hydroxides or sulfides, in warm soln of tartaric acid, or of bitartrates. LD50 orally in rats: >20 g/kg (Smyth).
Melting point: mp 655°
Boiling point: bp 1425°; bp210 870°
Toxicity data: LD50 orally in rats: >20 g/kg (Smyth)
Use: Manuf tartar emetic; as paint pigment; in enamels and glasses; as mordant; in flame-proofing canvas.