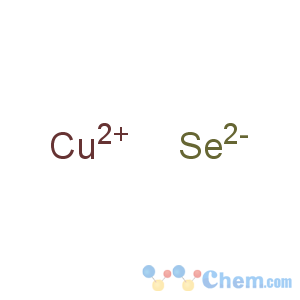
Title: Cupric Selenide
CAS Registry Number: 1317-41-5
Molecular Formula: CuSe
Molecular Weight: 142.51
Percent Composition: Cu 44.59%, Se 55.41%
Literature References: Occurs in nature as the mineral
klockmannite. Prepd by reduction of cupric selenite with hydrazine: Benzing
et al., J. Am. Chem. Soc. 80, 2657 (1958); Kulifay,
J. Inorg. Nucl. Chem. 25, 75 (1965).
Properties: Blue-black to greenish-black prismatic needles or hexagonal plates. Dec at a dull red heat. d 6.0-6.6. Sol in HCl with H2Se evolved, in H2SO4 with SO2 evolved; oxidized to CuSeO3 by HNO3.
Density: d 6.0-6.6
Use: Catalyst in Kjeldahl digestions; in semiconductors.