Title: Reinecke Salt
CAS Registry Number: 13573-16-5
CAS Name: Ammonium diamminetetrakis(thiocyanato-
N)chromate(1-)
Synonyms: ammonium reineckate; ammonium tetrathiocyanodiammonochromate
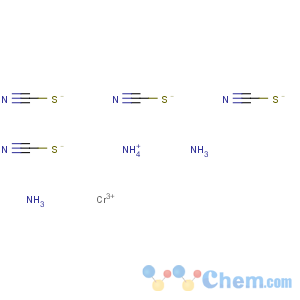
Molecular Formula: C4H10CrN7S4
Molecular Weight: 336.43
Percent Composition: C 14.28%, H 3.00%, Cr 15.46%, N 29.14%, S 38.12%
Line Formula: NH4[Cr(NH3)2(SCN)4]
Literature References: Exists as monohydrate; made by fusing ammonium thiocyanate with ammonium dichromate. Prepn: Dakin,
Org. Synth. coll. vol. II, 555 (1943).
Derivative Type: Monohydrate
Properties: Dark red crystals or red cryst powder. Sparingly sol in cold water; sol in hot water, alcohol. Dec in aq soln with formation of a blue color and free HCN; dec occurs in about 2 weeks at room temp, rapidly above 65°. A similar decompn takes place in boiling alcohol.
NOTE: The K-salt is also known as Reinecke salt.
Use: Precipitant for primary and secondary amines, proline, hydroxyproline, and certain amino acids; also a reagent for mercury with which it gives a red color or precipitate.