Title: Potassium Hexacyanocobaltate(III)
CAS Registry Number: 13963-58-1
CAS Name: Tripotassium hexakis(cyano-
C)cobaltate(3-)
Synonyms: potassium cyanocobaltate(III); potassium cobalticyanide; potassium cobaltihexacyanide; cobalt potassium cyanide
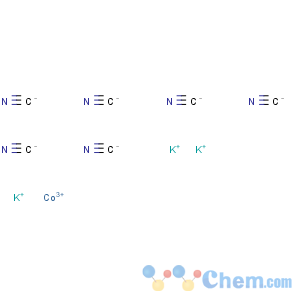
Molecular Formula: C6CoK3N6
Molecular Weight: 332.33
Percent Composition: C 21.68%, Co 17.73%, K 35.29%, N 25.29%
Line Formula: K3[Co(CN)6]
Literature References: Prepd by reacting cobalt acetate or cyanide with potassium cyanide, followed by air-oxidation of the cobaltocyanide formed: Biltz, Biltz,
Z. Anorg. Allg. Chem. 50, 108 (1906); Grube,
Z. Elektrochem. 32, 561 (1926); Benedetti-Pichler,
Z. Anal. Chem. 70, 258 (1927); Bigelow,
Inorg. Synth. 2, 225 (1946).
Properties: Faintly yellow, monoclinic crystals from water. d 1.906. Melts with decompn forming an olive-green mass. Freely sol in water, acetic acid solns. Insol in alcohol. Very slightly sol in liq ammonia. Dec by strong mineral acids. Aq solns are slightly yellow and are regarded as non-poisonous when freshly prepd: Zwenger,
Ann. 62, 178 (1847). An odor of HCN develops after about one week's standing in the dark. Exposure to light causes rapid development of a yellow color, but hydrolysis is slight.
Density: d 1.906