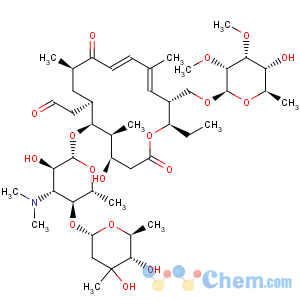
Title: Tylosin
CAS Registry Number: 1401-69-0
Trademarks: Tylan (Elanco)
Molecular Formula: C46H77NO17
Molecular Weight: 916.10
Percent Composition: C 60.31%, H 8.47%, N 1.53%, O 29.69%
Literature References: Macrolide antibiotic isolated from a strain of
Streptomycetes fradiae found in soil from Thailand: Hamill
et al., Antibiot. Chemother. 11, 328 (1961);
eidem, US 3178341 (1965 to Lilly). Prodn in batch and chemostat cultures: P. P. Gray, S. Bhuwapathanapun,
Biotechnol. Bioeng. 22, 1785 (1980). Partial structure: Morin, Gorman,
Tetrahedron Lett. 1964, 2339. Structure: Morin
et al., ibid. 1970, 4737; Achenbach
et al., Ber. 108, 2481 (1975). Configurational study: S. Omura
et al., Tetrahedron Lett. 1977, 1045. Abs config:
eidem, J. Antibiot. 33, 915 (1980); N. D. Jones
et al., ibid. 35, 420 (1982). Synthesis of
tylonolide, the aglycone: S. Masamune
et al., J. Am. Chem. Soc. 98, 7874 (1976); K. Tatsuta
et al., Tetrahedron Lett. 22, 3997 (1981). Relationship of ribosomal binding and antibacterial properties: J. W. Corcoran
et al., J. Antibiot. 30, 1012 (1977). Biosynthesis studies: E. T. Seno
et al., Antimicrob. Agents Chemother. 11, 455 (1977); S. Omura
et al., J. Antibiot. 31, 254 (1978).
Properties: Crystals from water, mp 128-132°. [a]D25 -46° (c = 2 in methanol). uv max: 282 nm (E1%1cm 245). Soly in water at 25°: 5 mg/ml. Sol in lower alcohols, esters and ketones, in chlorinated hydrocarbons, benzene, ether. Solns are stable at pH 4-9; at pH <4 another active compd,
desmycosin is formed.
Melting point: mp 128-132°
Optical Rotation: [a]D25 -46° (c = 2 in methanol)
Absorption maximum: uv max: 282 nm (E1%1cm 245)
Derivative Type: Hydrochloride
Molecular Formula: C46H77NO17.HCl
Molecular Weight: 952.56
Percent Composition: C 58.00%, H 8.25%, N 1.47%, O 28.55%, Cl 3.72%
Properties: Crystals from ethanol + ether, mp 141-145°.
Melting point: mp 141-145°
Therap-Cat-Vet: Antibacterial.