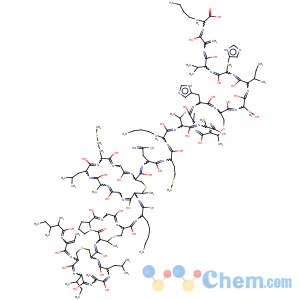
Title: Nisin
CAS Registry Number: 1414-45-5
CAS Name: Nisin A
Trademarks: Nisaplin (Aplin & Barrett); Novasin (Danisco)
Molecular Formula: C143H230N42O37S7
Molecular Weight: 3354.07
Percent Composition: C 51.21%, H 6.91%, N 17.54%, O 17.65%, S 6.69%
Literature References: Ribosomally synthesized antimicrobial peptide produced by
Lactococcus lactis (formerly
Streptococcus lactis); name derived from "group N inhibitory substance". Member of the class I bacteriocins known as lantibiotics; structure contains 34 amino acid residues, eight of which are rarely found in nature, including lanthionine,
q.v., and b-methyllanthionine. Isoln and antibacterial activity: A. T. R. Mattick, A. Hirsch,
Nature 154, 551 (1944);
eidem, Lancet 250, 417 (1946);
253, 5 (1947). Purification and nature of nisin: N. J. Berridge
et al., Biochem. J. 52, 529 (1952). Production: H. B. Hawley, R. H. Hall,
US 2935503 (1960 to Aplin & Barrett). Structure: E. Gross, J. L. Morrell,
J. Am. Chem. Soc. 93, 4634 (1971). Confirmation of structure of nisin and its major degradation product: M. Barber
et al., Experientia 44, 266 (1988). Partial synthesis: K. Fukase
et al., Bull. Chem. Soc. Jpn. 59, 2505 (1986). Total synthesis: K. Fukase
et al., Tetrahedron Lett. 29, 795 (1988). Biosynthetic study: G. W. Buchman
et al., J. Biol. Chem. 263, 16260 (1988). Review of properties and commercial applications: A. Hurst, D. G. Hoover,
Food Sci. Technol. 57, 369-394 (1993); J. Delves-Broughton
et al., Antonie van Leeuwenhoek 69, 193-202 (1996); of biosynthesis: C.-I. Cheigh, Y.-R. Pyun,
Biotechnol. Lett. 27, 1641-1648 (2005); of biosynthesis, mode of action and comparison with other lantibiotics: C. Chatterjee
et al., Chem. Rev. 105, 633-683 (2005).
Properties: Crystals from ethanol. Soly in dilute HCl: 12% (pH 2.5); 4% (pH 5.0). Stable to boiling in acid soln.
Derivative Type: Nisin Z
CAS Registry Number: 137061-46-2
Synonyms: 27-L-Asparagine-nisin
Molecular Formula: C141H229N41O38S7
Molecular Weight: 3331.03
Percent Composition: C 50.84%, H 6.93%, N 17.24%, O 18.25%, S 6.74%
Literature References: Naturally occurring nisin variant. Identification: J. W. Mulders
et al., Eur. J. Biochem. 201, 581 (1991).
Use: Food preservative, esp. for cheese and other dairy products, canned vegetables, and some baked goods.