Title: Iminodiacetic Acid
CAS Registry Number: 142-73-4
CAS Name: N-(Carboxymethyl)glycine
Synonyms: iminodiethanoic acid; diglycine; IDA
Molecular Formula: C4H7NO4
Molecular Weight: 133.10
Percent Composition: C 36.10%, H 5.30%, N 10.52%, O 48.08%
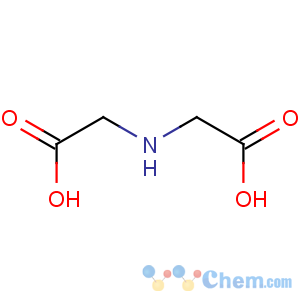
Line Formula: HOOCCH2NHCH2COOH
Literature References: Obtained from nitrilotriacetic acid, N(CH2COOH)3, by HCl-hydrolysis in a bomb tube: Schwarzenbach
et al., Helv. Chim. Acta 28, 1133 (1945); by oxygenation in presence of palladium/carbon catalyst: Tetenbaum, Stone,
Chem. Commun. 1970, 1699. Iminodiacetic acid and nitrilotriacetic acid are formed upon boiling chloroacetic acid with concd aq ammonia: Heintz,
Ann. 149, 88 (1869).
See also Martell, Bersworth,
J. Org. Chem. 15, 46 (1950).
Properties: Orthorhombic crystals, dec 247.5° (commercial grade, mp 220-250°). pKa1 2.98; pKa2 9.89. Forms salts with acids and bases. Soly in water at 5°: 2.43 g/100 ml. Practically insol in acetone, methanol, ether, benzene, carbon tetrachloride, heptane.
Melting point: mp 220-250°
pKa: pKa1 2.98; pKa2 9.89
Derivative Type: Hydrochloride
Molecular Formula: C4H7NO4.HCl
Molecular Weight: 169.56
Percent Composition: C 28.33%, H 4.76%, N 8.26%, O 37.74%, Cl 20.91%
Properties: Crystals, dec 238-239°. Concd aq solns yield the free acid when adjusted to pH 2 with NaOH.
Derivative Type: Sodium salt monohydrate
Molecular Formula: C4H6NNaO4.H2O
Molecular Weight: 173.10
Percent Composition: C 27.75%, H 4.66%, N 8.09%, Na 13.28%, O 46.21%
Properties: Freely sol in water. Forms complexes with Mg, Ca, Ba.
Use: Has been suggested as intermediate in the manuf of surface active agents, complex salts, chelating agents.