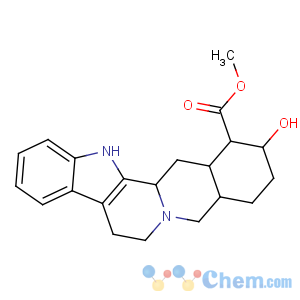
Title: Yohimbine
CAS Registry Number: 146-48-5
CAS Name: (16a,17a)-17-Hydroxyyohimban-16-carboxylic acid methyl ester
Synonyms: quebrachine; corynine; aphrodine
Molecular Formula: C21H26N2O3
Molecular Weight: 354.44
Percent Composition: C 71.16%, H 7.39%, N 7.90%, O 13.54%
Literature References: Indole alkaloid with a2-adrenergic blocking activity. Found in
Corynanthe johimbe K. Schum.,
Rubiaceae and related trees, also in
Rauwolfia serpentina (L.) Benth.,
Apocynaceae: Raymond-Hamet,
J. Pharm. Chim. 19, 209 (1934); Hofmann,
Helv. Chim. Acta 37, 849 (1954); Stoll, Jucker,
Ullmanns Encyklop?die der technischen Chemie vol. 3 (Munich, 3rd ed., 1953) p 266; Bader
et al., J. Am. Chem. Soc. 76, 1695 (1954). Structure: Witkop,
Ann. 554, 83 (1943); Clemo, Swan,
J. Chem. Soc. 1946, 617. Stereochemistry: Janot
et al., Bull. Soc. Chim. Fr. 1952, 1085; Godfredsen, Vandegal,
Acta Chem. Scand. 10, 1414 (1956); Van Tamelen
et al., J. Am. Chem. Soc. 78, 4628 (1956); Ban, Yonemitsu,
Tetrahedron 20, 2877 (1964). Synthesis: Van Tamelen
et al., J. Am. Chem. Soc. 80, 5006 (1958); Liljegren, Potts,
J. Org. Chem. 27, 377 (1962). Total synthesis: Van Tamelen
et al., J. Am. Chem. Soc. 91, 7315 (1969); Stork, Guthikonda,
ibid. 94, 5109 (1972); T. Kametani
et al., Chem. Pharm. Bull. 24, 2500 (1976); E. Wenkert
et al., J. Am. Chem. Soc. 100, 4894 (1978);
101, 5370 (1979);
104, 2244 (1982); I. Ninomiya
et al., Heterocycles 14, 631 (1980). Pharmacokinetics in humans: J. A. Owen
et al., Eur. J. Clin. Pharmacol. 32, 577 (1987). Clinical studies in impotence: K. Reid
et al., Lancet 2, 421 (1987); A. Morales
et al., J. Urol. 137, 1168 (1987). Review of pharmacology and use in molecular studies of a2-adrenoreceptor: M. R. Goldberg, D. Robertson,
Pharmacol. Rev. 35, 143-180 (1987). Comprehensive description: A. G. Mekkawi, A. A. Al-Badr,
Anal. Profiles Drug Subs. 16, 731-768 (1986).
Properties: Orthorhombic needles from dil alc, mp 234°. Also mp 235-237°. [a]D20 +50.9 to +62.2° (ethanol); [a]D20 +108° (pyridine); [a]20546 +129° (c = 0.5 in pyridine). uv max (methanol): 226, 280, 291 nm (log e 4.56, 3.88, 3.80). Sparingly sol in water. Sol in alcohol, chloroform, hot benzene; moderately sol in ether.
Melting point: mp 234°; mp 235-237°
Optical Rotation: [a]D20 +50.9 to +62.2° (ethanol); [a]D20 +108° (pyridine); [a]20546 +129° (c = 0.5 in pyridine)
Absorption maximum: uv max (methanol): 226, 280, 291 nm (log e 4.56, 3.88, 3.80)
Derivative Type: Hydrochloride
CAS Registry Number: 65-19-0
Trademarks: Antagonil (Wildlife Pharm.); Aphrodyne (Star); Erex (Ion); Yobine (Lloyd); Yocon (Palisades); Yohimex (Kramer); Yohydrol (Riedel-Zabinka); Yovital (Kenwood)
Molecular Formula: C21H26N2O3.HCl
Molecular Weight: 390.90
Percent Composition: C 64.52%, H 6.96%, N 7.17%, O 12.28%, Cl 9.07%
Properties: Orthorhombic plates, prisms from alc; dec 302°. [a]D22 +105° (H2O). Sol in ~120 ml water, 400 ml alc. The aq soln is about neutral.
Optical Rotation: [a]D22 +105° (H2O)
Use: Pharmacological probe for the study of a2-adrenoceptor.
Therap-Cat: Mydriatic. In treatment of impotence.
Therap-Cat-Vet: Xylazine reversing agent.
Keywords: a-Adrenergic Blocker; Mydriatic.