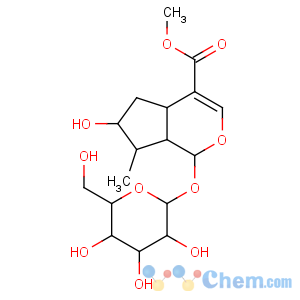
Title: Loganin
CAS Registry Number: 18524-94-2
CAS Name: 1-(b-D-Glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methylcyclopenta[
c]pyran-4-carboxylic acid methyl ester
Synonyms: 7-hydroxy-6-desoxyverbenalin
Molecular Formula: C17H26O10
Molecular Weight: 390.38
Percent Composition: C 52.30%, H 6.71%, O 40.98%
Literature References: Key intermediate in the biosynthesis of indole alkaloids. First isolated from the seeds but chiefly from the pulp of the fruit of
Strychnos nux-vomica L.,
Loganiaceae: Dunstan, Short,
Pharm. J. 14, 1025 (1883); Merz, Krebs,
Arch. Pharm. 275, 217 (1937); Merz, Lehmann,
ibid. 290, 543 (1957). Structure: Sheth
et al., Tetrahedron Lett. 1961, 394; Büchi, Manning,
Tetrahedron 18, 1049 (1962). Crystal structure: Lentz, Rossmann,
Chem. Commun. 1969, 1269; P. G. Jones
et al., Acta Crystallogr. B36, 481 (1980). Abs config: Inouye
et al., Tetrahedron 26, 3905 (1970). Total synthesis: Büchi
et al., J. Am. Chem. Soc. 92, 2165 (1970); Partridge
et al., ibid. 95, 532 (1973); Büchi
et al., ibid. 540; B.-W. Au-Yeung, I. Fleming,
Chem. Commun. 1977, 81; I. Fleming, B.-W. Au-Yeung,
Tetrahedron 37, Suppl. 9, 13 (1981); K. Hiroi
et al., Chem. Lett. 1981, 559. Biosynthetic studies: Battersby, "Biosynthesis II: Terpenoid Indole Alkaloids", in
The Alkaloids vol. 1, The Chemical Society (Burlington House, London, 1971) pp 31-47.
Properties: Crystals, mp 222-223°. [a]D20 -82.1° (water). Freely sol in water; less sol in 96% alcohol; sparingly in abs alcohol. Practically insol in ether, petr ether, ligroin, ethyl acetate, acetone, chloroform.
Melting point: mp 222-223°
Optical Rotation: [a]D20 -82.1° (water)