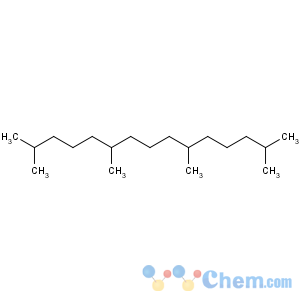
Title: Pristane
CAS Registry Number: 1921-70-6
CAS Name: 2,6,10,14-Tetramethylpentadecane
Synonyms: norphytane
Trademarks: Robuoy (Robeco)
Molecular Formula: C19H40
Molecular Weight: 268.52
Percent Composition: C 84.99%, H 15.01%
Literature References: Isoprenoid alkane obtained from the unsaponifiable fraction of shark liver oil where it occurs to an extent of 14%: Tsujimoto,
J. Soc. Chem. Ind. 51, 317T (1932); S?rensen, Mehllum,
Acta Chem. Scand. 2, 140 (1948). Identity with norphytane: Pliva, S?rensen,
ibid. 4, 846 (1950). Isoln from petroleum crude oils: Bendoraitis
et al., Anal. Chem. 34, 49 (1962); from wool wax: Mold
et al., Nature 199, 283 (1963). Synthesis from phytol: S?rensen, S?rensen,
Acta Chem. Scand. 3, 939 (1949). Metabolism: McKenna, Kallio,
Proc. Natl. Acad. Sci. USA 68, 1552 (1971). Use in inducing murine plasmacytomas: P. N. Anderson, M. Potter,
Nature 222, 994 (1969). Effect on ascites tumor formation and monoclonal antibody production: N. J. Hoogenraad, C. J. Wraight,
Methods Enzymol. 121, 375 (1986).
Properties: Mobile, transparent, stable liq. d420 0.78267. Congealing point -100°. bp760 296°; bp10 158°; bp0.001 68° (bath temp).
nD20 1.43848. Acid no. 0-5. Iodine no. 0-7.5. Sapon no. 0-5. Viscosity at 25°: 5 cP. Soluble in ether, petr ether, benzene, chloroform, carbon tetrachloride.
Boiling point: bp760 296°; bp10 158°; bp0.001 68° (bath temp)
Index of refraction: nD20 1.43848
Density: d420 0.78267
Use: Lubricant; transformer oil. Anti-corrosion agent. Biological marker. In experimental systems to induce plasmacytomas; in production of monoclonal antibodies.