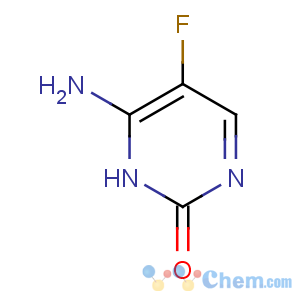
Title: Flucytosine
CAS Registry Number: 2022-85-7
CAS Name: 4-Amino-5-fluoro-2(1
H)-pyrimidinone
Synonyms: 5-fluorocytosine; 5-FC
Manufacturers' Codes: Ro-2-9915
Trademarks: Alcobon (Roche); Ancobon (Roche); Ancotil (Roche)
Molecular Formula: C4H4FN3O
Molecular Weight: 129.09
Percent Composition: C 37.22%, H 3.12%, F 14.72%, N 32.55%, O 12.39%
Literature References: Prepn: Duschinsky
et al., J. Am. Chem. Soc. 79, 4559 (1957); Heidelberger, Duschinsky,
US 2802005 (1957); Duschinsky, Heidelberger,
US 2945038 and Duschinsky,
US 3040026 (1960, 1962 both to Hoffmann-La Roche); Undheim, Gacek,
Acta Chem. Scand. 23, 294 (1969). Patents as a fungicide: Berger, Duschinsky,
BE 628615 and
US 3368938 (1963, 1968 both to Hoffmann-La Roche). Activity studies: Grunberg
et al., Antimicrob. Agents Chemother. 1963, 566; Shadomy
et al., ibid. 1968, 452; Grunberg
et al., in
5th Int. Congr. Chemother., Proc. vol. IV, K. Spitzy, Ed. (Verlag Wiener Med. Akad., 1967, Austria) p 69. Metabolic studies: Koechlin
et al., Biochem. Pharmacol. 15, 435 (1966). Clinical results: Utz
et al., Antimicrob. Agents Chemother. 1968, 344; Warner
et al., ibid. 1970, 473. Comprehensive description: E. H. Waysek, J. H. Johnson,
Anal. Profiles Drug Subs. 5, 115-138 (1976).
Properties: Odorless, white crystalline solid, mp 295-297° (dec). uv max (0.1
N HCl): 285 nm (e 8900). Soly in water: 1.5 g/100 ml at 25°C. pKa1: 3.26. LD50 in mice (mg/kg): >2000 orally and s.c.; 1190 i.p.; 500 i.v. (Grunberg, 1963).
Melting point: mp 295-297° (dec)
pKa: pKa1: 3.26
Absorption maximum: uv max (0.1
N HCl): 285 nm (e 8900)
Toxicity data: LD50 in mice (mg/kg): >2000 orally and s.c.; 1190 i.p.; 500 i.v. (Grunberg, 1963)
Therap-Cat: Antifungal.
Keywords: Antifungal (Synthetic).