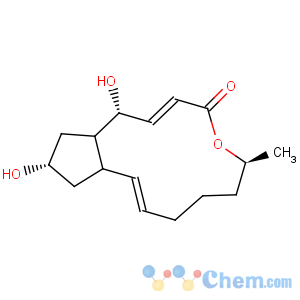
Title: Brefeldin A
CAS Registry Number: 20350-15-6
CAS Name: 1,6,7,8,9,11a,12,13,14,14a-Decahydro-1,13-dihydroxy-6-methyl-4
H-cyclopent[
f]oxacyclotridecin-4-one
Synonyms: g,4-dihydroxy-2-(6-hydroxy-1-heptenyl)-4-cyclopentanecrotonic acid l-lactone; ascotoxin; cyanein; decumbin
Molecular Formula: C16H24O4
Molecular Weight: 280.36
Percent Composition: C 68.54%, H 8.63%, O 22.83%
Literature References: A fungal metabolite which is a macrocyclic lactone exhibiting a wide range of antibiotic activity. Produced by
Penicillium brefeldianum Dodge: E. Haerri
et al., Helv. Chim. Acta 46, 1235 (1963). Also produced by
P. decumbens: V. L. Singleton
et al., Nature 181, 1072 (1958);
P. cyaneum: V. Betina
et al., Folia Microbiol. 7, 353 (1962). Structure: H. P. Sigg,
Helv. Chim. Acta 47, 1401 (1964). Abs configuration: H. P. Weber
et al., ibid. 54, 2763 (1971). Synthesis of (±)-form: E. J. Corey, R. H. Wollenberg,
Tetrahedron Lett. 1976, 4705; E. J. Corey
et al., ibid. 1977, 2243; R. Baudouy
et al., ibid. 2973; P. A. Bartlett, F. R. Green,
J. Am. Chem. Soc. 100, 4548 (1978); A. E. Greene
et al., ibid. 102, 7583 (1980); M. Honda
et al., Tetrahedron Lett. 1981, 2679. Total synthesis of (+)-form: T. Kitahara
et al., ibid. 1979, 3021. Biosynthesis: B. E. Cross, P. Hendley,
Chem. Commun. 1975, 124; C. R. Hutchinson
et al., J. Am. Chem. Soc. 103, 2474, 2477 (1981); M. Sunagawa
et al., J. Antibiot. 36, 25 (1983). Antifungal activity: V. Betina
et al., ibid. 17A, 93 (1964); anti-HeLa cell effect:
eidem, Naturwissenschaften 49, 241 (1962).
See also W. Keller-Schierlein, "Chemistry of Macrolide Antibiotics" in
Fortschr. Chem. Org. Naturst. 30, 313-445 (1973).
Properties: Colorless prisms from methanol/ether, mp 204-205°. uv max (ethanol): 215 nm (log e 4.05). [a]D22 +96 ±2° (c = 1.08 in methanol). LD50 i.p. in mice: >200 mg/kg (Haerri).
Melting point: mp 204-205°
Optical Rotation: [a]D22 +96 ±2° (c = 1.08 in methanol)
Absorption maximum: uv max (ethanol): 215 nm (log e 4.05)
Toxicity data: LD50 i.p. in mice: >200 mg/kg (Haerri)