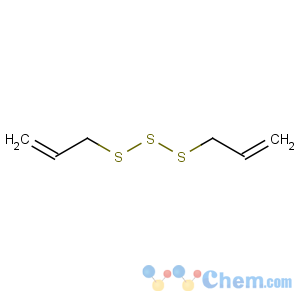
Title: Diallyl Trisulfide
CAS Registry Number: 2050-87-5
CAS Name: Di-2-propenyl trisulfide
Synonyms: allyl trisulfide
Trademarks: DATS
Molecular Formula: C6H10S3
Molecular Weight: 178.34
Percent Composition: C 40.41%, H 5.65%, S 53.94%
Line Formula: (CH2=CHCH2S)2S
Literature References: Component of the essential oil of garlic; formed by pyrolysis of diallyl disulfide. Major component of the Chinese traditional medicine,
allitridium. Exhibits antifungal, antitumor and antioxidant activity. Isoln from garlic oil: F. W. Semmler,
Arch. Pharm. 230, 434 (1892); E. Block
et al., J. Am. Chem. Soc. 110, 7813 (1988). Synthesis: B. Milligan
et al., J. Chem. Soc. 1961, 4850; A. Banerji, G. P. Kalena,
Tetrahedron Lett. 21, 3003 (1980). Inhibitory effect on mammary tumor cells
in vitro: S. G. Sundaram, J. A. Milner,
Cancer Lett. 74, 85 (1993). Mechanism of action study: X. Hu
et al., Arch. Biochem. Biophys. 336, 199 (1996). Antifungal activity: J. Shen
et al., Planta Med. 62, 415 (1996).
Properties: Oil. bp6 92°; bp0.0008 66-67°.
nD20 1.5896.
Boiling point: bp6 92°; bp0.0008 66-67°
Index of refraction: nD20 1.5896