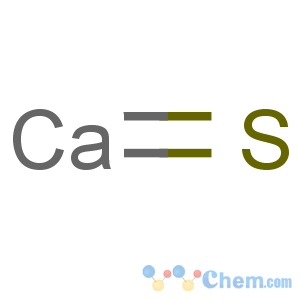Title: Calcium Sulfide
CAS Registry Number: 20548-54-3
Molecular Formula: CaS
Molecular Weight: 72.14
Percent Composition: Ca 55.56%, S 44.45%
Literature References: Pure CaS prepd in the laboratory by heating pure CaCO3 in a stream of H2S + H2 at 1000°: Ehrlich in
Handbook of Preparative Inorganic Chemistry vol. 1, G. Brauer, Ed. (Academic Press, New York, 2nd ed., 1963) p 938. Crude calcium sulfide, erroneously called
sulfurated lime,
calcic liver of sulfur,
liver of lime,
hepar calcis, made by igniting calcium sulfate with carbonaceous matter. Contains not less than 55% CaS; the balance is calcium sulfate, sulfite and carbonate, and the "ash" from the carbonaceous material.
See Mellor's vol III, p 740 (1928).
Luminous calcium sulfide or
Canton's phosphorus made by igniting a mixture of CaCO3 and S with very small quantities of Bi or Mn salts, etc.: Verneuil,
Compt. Rend. 103, 600 (1886);
Mellor's, loc. cit.
Properties: White powder if pure; crude and luminous calcium sulfide may be yellowish to pale-gray. Odor of H2S in moist air; unpleasant alkaline taste. Oxidizes in dry air and dec in moist air. mp >2000°. d 2.59. Slightly sol in cold, more sol in hot water with partial decompn; freely sol in solns of ammonium salts; practically insol in alcohol; dec even by weak acids, evolving H2S.
Keep well closed.
Melting point: mp >2000°
Density: d 2.59
Use: In phosphors; as lubricant additive. Pure CaS used in electron emitters. Luminous CaS used for making luminous paints or varnishes.
Therap-Cat-Vet: Has been used in chronic suppurative lesions.