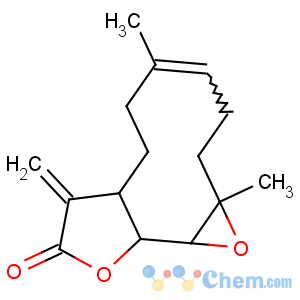
Title: Parthenolide
CAS Registry Number: 20554-84-1
CAS Name: (1a
R,4
E,7a
S,10a
S,10b
S)-2,3,6,7,7a,8,10a,10b-Octahydro-1a,5-dimethyl-8-methyleneoxireno[9,10]cyclodeca[1,2-
b]furan-9(1a
H)-one
Synonyms: 4,5a-epoxy-6b-hydroxy-germacra-1(10),11(13)-dien-12-oic acid g-lactone
Molecular Formula: C15H20O3
Molecular Weight: 248.32
Percent Composition: C 72.55%, H 8.12%, O 19.33%
Literature References: Sesquiterpene lactone found in feverfew,
q.v., and in other plants. Isolation from
Chrysanthemum parthenium (L.) Bernh.
Compositae and characterization: V. Herout
et al., Chem. Ind. (London) 1959, 1069; M. Soucek
et al., Collect. Czech. Chem. Commun. 26, 803 (1961); from
Magnolia grandiflora L.,
Magnoliaceae: F. S. El-Feraly, Y.-M. Chan,
J. Pharm. Sci. 67, 347 (1978). Revised structure and spectral analysis: T. R. Govindachari
et al., Tetrahedron 21, 1509 (1965). Absolute configuration: A. S. Bawdekar
et al., Tetrahedron Lett. 1966, 1225. Crystal structure: A. Quick, D. Rogers,
J. Chem. Soc. Perkin Trans. 2 4, 465 (1976). HPLC determn: D. Strack
et al., Z. Naturforsch. 35, 915 (1980). Cytotoxicity: K.-H. Lee
et al., Cancer Res. 31, 1649 (1971); L. A. J. O'Neill
et al., Br. J. Clin. Pharmacol. 23, 81 (1987).
Properties: Colorless plates, mp 115-116°. [a]D20 -81.4° (c = 1.04 in chloroform); [a]D22 -71.4° (c = 0.220 in CH2Cl2). uv max: 214 nm (log e 4.22).
Melting point: mp 115-116°
Optical Rotation: [a]D20 -81.4° (c = 1.04 in chloroform); [a]D22 -71.4° (c = 0.220 in CH2Cl2)
Absorption maximum: uv max: 214 nm (log e 4.22)