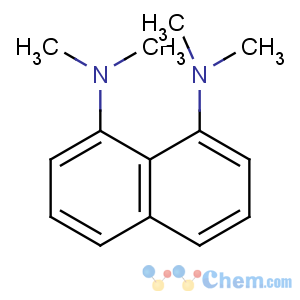
Title: DMAN
CAS Registry Number: 20734-58-1
CAS Name: N,
N,
N¢,
N¢-Tetramethyl-1,8-naphthalenediamine
Synonyms: 1,8-bis(dimethylamino)naphthalene; proton sponge
Molecular Formula: C14H18N2
Molecular Weight: 214.31
Percent Composition: C 78.46%, H 8.47%, N 13.07%
Literature References: Prototype of a class of neutral aromatic diamines with exceptionally high basicity known as proton sponges. Prepn: W. G. Brown, N. J. Letang,
J. Am. Chem. Soc. 63, 358 (1941); and basicity study: R. W. Alder
et al., J. Chem. Soc. Chem. Commun. 1968, 723. X-ray crystal structure: H. Einspahr
et al., Acta Crystallogr. B29, 1611 (1973). Proton transfer studies: F. Hibbert,
J. Chem. Soc. Perkin Trans. 2 1974, 1862.
Ab initio structural studies: J. A. Platts
et al., J. Org. Chem. 59, 4647 (1994); and fluorescence spectroscopy: A. Szemik-Hojniak
et al., J. Am. Chem. Soc. 120, 4840 (1998). Charge distribution: P. R. Mallinson
et al., ibid. 121, 4640 (1999). 13C NMR study of hydrogen bonding: M. Pietrzak
et al., ibid. 123, 4338 (2001). Catalytic activity: I. Rodriguez
et al., J. Catal. 183, 14 (1999). Review of chemistry: H. A. Staab, T. Saupe,
Angew. Chem. Int. Ed. 27, 865-879 (1988); R. W. Alder,
Chem. Rev. 89, 1215-1223 (1989).
Properties: bp4 144-145°. mp 47-48°. d 1.12. Absorption max: 335 nm (log e 3.96). pKa 12.1.
Melting point: mp 47-48°
Boiling point: bp4 144-145°
pKa: pKa 12.1
Absorption maximum: Absorption max: 335 nm (log e 3.96)
Density: d 1.12
Use: Very strong base in organic synthesis and catalysis.