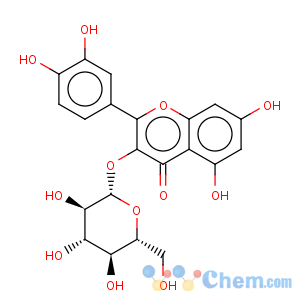
Title: Isoquercitrin
CAS Registry Number: 21637-25-2
CAS Name: 2-(3,4-Dihydroxyphenyl)-3-(b-D-glucofuranosyloxy)-5,7-dihydroxy-4
H-1-benzopyran-4-one
Synonyms: 3,3¢,4¢,5,7-pentahydroxyflavone-3-glucoside; quercetin-3-glucoside; isotrifoliin; trifoliin
Molecular Formula: C21H20O12
Molecular Weight: 464.38
Percent Composition: C 54.31%, H 4.34%, O 41.34%
Literature References: From flowers of
Gossypium herbaceum L.,
Malvaceae: Perkin,
J. Chem. Soc. 95, 2181 (1909); from flowers of
Aesculus hippocastanum L.,
Hippocastanaceae: H?rhammer, Wagner,
Arch. Pharm. 290, 224 (1957); from
Tropaeolum majus L.,
Tropaeolaceae: Delaveau,
Compt. Rend. 252, 1510 (1961); from
Arnica montana L.,
Compositae: Friedrick,
Naturwissenschaften 49, 541 (1962). Structure: Attree, Perkin,
J. Chem. Soc. 1927, 234. Identity with trifoliin and isotrifoliin: Hattori
et al., J. Chem. Soc. Jpn. 58, 844 (1937),
C.A. 32, 219c (1938). Synthesis: Ice, Wender,
J. Am. Chem. Soc. 74, 4606 (1952);
eidem, US 2727890 (1955 to the USAEC).
Properties: Yellow needles from water, mp 225-227°. uv max: 257, 369 nm. Practically insol in cold but sparingly sol in boiling water. Sol in alkaline solns with a deep yellow tint.
Melting point: mp 225-227°
Absorption maximum: uv max: 257, 369 nm