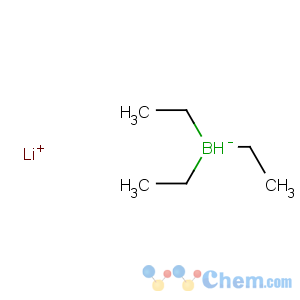
Title: Lithium Triethylborohydride
CAS Registry Number: 22560-16-3
CAS Name: (
T-4)-Lithium triethylhydroborate(1-)
Trademarks: Super-Hydride (Aldrich)
Molecular Formula: C6H16BLi
Molecular Weight: 105.94
Percent Composition: C 68.02%, H 15.22%, B 10.20%, Li 6.55%
Line Formula: LiBH(C2H5)3
Literature References: Commercial product is available in THF solution. Prepn: H. C. Brown
et al., J. Am. Chem. Soc. 75, 192 (1953); P. Binger
et al., Ann. 717, 21 (1968); H. C. Brown
et al., Inorg. Chem. 16, 2229 (1977). Mechanism of reduction of organic halides: E. C. Ashby
et al., J. Org. Chem. 49, 4505 (1984). Use as a reducing agent in organic and inorganic synthesis: H. C. Brown
et al., J. Org. Chem. 45, 1 (1980); S. Krishnamurthy, H. C. Brown,
ibid. 48, 3085 (1983); H. C. Brown, S.-C. Kim,
ibid. 49, 1064 (1984); B. E. Blough, F. I. Carroll,
Tetrahedron Lett. 34, 7239 (1993); C. K. Yee
et al., Langmuir 15, 3486 (1999); H. Tanaka, K. Ogasawara,
Tetrahedron Lett. 43, 4417 (2002).
Properties: Colorless crystals, mp 66-67° (Binger). Also reported as white needles from benzene, mp 78-83° (dec) (Brown, 1977). Moderately sol in benzene, toluene,
n-hexane.
Unstable in air. Reacts violently with water.
Melting point: mp 66-67° (Binger); mp 78-83° (dec) (Brown, 1977)
Use: Powerful and selective reducing agent.