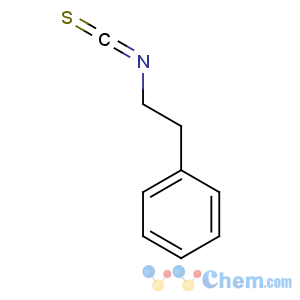
Title: Phenethyl Isothiocyanate
CAS Registry Number: 2257-09-2
CAS Name: (2-Isothiocyanatoethyl)benzene
Synonyms: 2-phenylethyl isothiocyanate; PEITC
Molecular Formula: C9H9NS
Molecular Weight: 163.24
Percent Composition: C 66.22%, H 5.56%, N 8.58%, S 19.64%
Literature References: Chemopreventive agent found in cruciferous vegetables; produced by hydrolysis of
gluconasturtiin. Prepn: J. v. Braun, H. Deutsch,
Ber. 45, 2188 (1912); E. Schmidt
et al., Ann. 612, 11 (1958); and isoln from turnips: E. P. Lichtenstein
et al., J. Agric. Food Chem. 10, 30 (1962). Isoln from cabbage and watercress seeds: N. Kaoulla
et al., Phytochemistry 19, 1053 (1980). LC/MS determn in plasma and urine: Y. Ji, M. E. Morris,
Anal. Biochem. 323, 39 (2003). Chemopreventive studies in animal models: G. D. Stoner,
Curr. Top. Plant Physiol. 15, 78 (1995); A. Nishikawa
et al., Curr. Cancer Drug Targets 4, 373 (2004). Mechanistic studies of anticancer activity and induction of apoptosis: Y.-R. Chen
et al., J. Biol. Chem. 277, 39334 (2002); R. Hu
et al., Carcinogenesis 24, 1361 (2003); D. Xiao
et al., Clin. Cancer Res. 11, 2670 (2005).
Properties: Colorless to pale yellow liquid, bp11 139-140°. bp1.4-1.5 102-103°.
nD20 1.5904. d420 1.0942. LD50 in mice (mg/kg): 700 orally; 150 s.c.; 50 i.v. (Lichtenstein).
Boiling point: bp11 139-140°; bp1.4-1.5 102-103°
Index of refraction: nD20 1.5904
Density: d420 1.0942
Toxicity data: LD50 in mice (mg/kg): 700 orally; 150 s.c.; 50 i.v. (Lichtenstein)
Use: Flavor ingredient; provides the hot or burning sensation in horseradish.