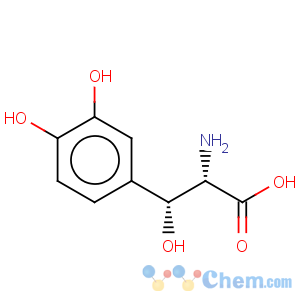
Title: Droxidopa
CAS Registry Number: 23651-95-8
CAS Name: threo-b,3-Dihydroxy-L-tyrosine
Synonyms: L-
threo-3-(3,4-dihydroxyphenyl)serine; (-)-(2
S,3
R)-2-amino-3-hydroxy-3-(3,4-dihydroxyphenyl)propionic acid;
threo-dopaserine; L-
threo-DOPS; L-DOPS
Manufacturers' Codes: SM-5688
Trademarks: Dops (Sumitomo)
Molecular Formula: C9H11NO5
Molecular Weight: 213.19
Percent Composition: C 50.70%, H 5.20%, N 6.57%, O 37.52%
Literature References: Synthetic amino acid precursor of norepinephrine,
q.v. Prepn of racemate: K. W. Rosenmund, H. Dornsaft,
Ber. 52B, 1734 (1919). Separation and resolution of diastereomers: B. Hegedüs
et al., Helv. Chim. Acta 58, 147 (1975); B. Hegedüs, A. Krasso,
US 3920728 (1975 to Hoffmann-La Roche). Improved process for production: N. Ohashi
et al., US 4319040 (1982 to Sumitomo). Pharmacology of stereoisomers: G. Bartholini
et al., J. Pharmacol. Exp. Ther. 193, 523 (1975). Clinical pharmacology of L
-threo-form and clinical evaluation in familial amyloid polyneuropathy (FAP): T. Suzuki
et al., Eur. J. Clin. Pharmacol. 17, 429 (1980). Reversed-phase chromatography determn in plasma and urine: F. Boomsma
et al., J. Chromatogr. 427, 219 (1988). Pharmacokinetics in FAP: T. Suzuki
et al., Eur. J. Clin. Pharmacol. 23, 463 (1982); in parkinsonism: T. Suzuki
et al., Neurology 34, 1446 (1984). Metabolism to norepinephrine: T. Suzuki
et al., Life Sci. 36, 435 (1985). Clinical studies in Parkinson's disease: N. Ogawa
et al., J. Med. 16, 525 (1985); H. Narabayashi
et al., Adv. Neurol. 45, 593 (1986).
Properties: Crystals from ethanol and ether, mp 232-235° (dec). [a]D20 -39° (c = 1 in 1
N aq HCl). Also cited as crystals from water and L-ascorbic acid, mp 229-232° (dec) (Ohashi). [a]D20 -42.0° (c = 1 in 1
N aq HCl).
Melting point: mp 232-235° (dec); mp 229-232° (dec) (Ohashi)
Optical Rotation: [a]D20 -39° (c = 1 in 1
N aq HCl); [a]D20 -42.0° (c = 1 in 1
N aq HCl)
Therap-Cat: Antiparkinsonian.
Keywords: Antiparkinsonian.