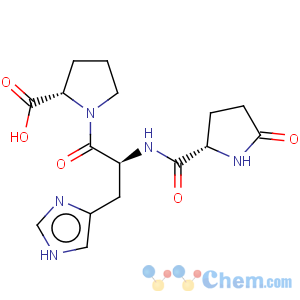
Title: TRH
CAS Registry Number: 24305-27-9
CAS Name: 5-Oxo-L-prolyl-L-histidyl-L-prolinamide
Synonyms: thyrotropin-releasing factor; thyrotropin releasing hormone; TRF; protirelin; lopremone (rescinded USAN); TSH-releasing factor; pyroglutamylhistidylprolinamide; thyroliberin
Trademarks: Antepan (Henning); Stimu-TSH (HMR); Thypinone (Abbott); Thyrefact (HMR)
Molecular Formula: C16H22N6O4
Molecular Weight: 362.38
Percent Composition: C 53.03%, H 6.12%, N 23.19%, O 17.66%
Literature References: A hypothalamic neurohormone which stimulates the release and synthesis of TSH,
q.v., from the anterior pituitary via the hypophyseal portal system; the first of the hypothalamic regulatory hormones to be isolated, characterized, and synthesized. Isoln from bovine hypothalami: Schreiber
et al., Experientia 18, 338 (1962); Schally
et al., Endocrinology 78, 726 (1966). Purification from ovine hypothalami: Guillemin
et al., C.R. Seances Acad. Sci. Ser. D 262, 2278 (1966). Isoln from porcine hypothalami and properties: Schally
et al., Biochem. Biophys. Res. Commun. 25, 165 (1966); Schally
et al., J. Biol. Chem. 244, 4077 (1969). Structural studies: Folkers
et al., Biochem. Biophys. Res. Commun. 37, 123 (1969); Burgus
et al., C.R. Seances Acad. Sci. Ser. D 269, 226 (1969). Identity of isolated TRH with synthetic tripeptide: Burgus
et al., ibid. 1870; Bowers
et al., Endocrinology 86, 573, 1143 (1970). Solubility: Burgus
et al., Experientia 23, 417 (1967). Synthesis: Flouret,
J. Med. Chem. 13, 843 (1970); Chang
et al., ibid. 14, 481 (1971); E. Gross
et al., Angew. Chem. Int. Ed. 12, 664 (1972); P. G. Pietta
et al., J. Org. Chem. 39, 44 (1974). Review of synthetic methods: Rivier,
Methods Enzymol. 37, 408 (1975). TRH is also believed to induce the secretion of the pituitary lactogenic hormone, prolactin,
q.v.: Tasjian
et al., Biochem. Biophys. Res. Commun. 43, 516 (1971); Bowers
et al., ibid. 51, 512 (1973). It has been shown to block and reverse leukotriene-induced hypotension in the unanesthetized guinea pig: W. E. Lux
et al., Nature 302, 822 (1983). Clinical studies: Hershman,
N. Engl. J. Med. 290, 886 (1974). Reviews of TRH and other hypothalamic releasing hormones: Schally
et al., Recent Prog. Horm. Res. 24, 497 (1968); Burgus, Guillemin,
Annu. Rev. Biochem. 39, 499 (1970);
Polypeptide Hormones, R. F. Beers, E. G. Bassett, Eds. (Raven Press, New York, 1980) pp 165-278.
Properties: Purified TRH is partially sol in chloroform, highly sol in absolute methanol. Completely insol in pyridine. Inactivated by diazotized sulfanilic acid (Pauly reagent) and by plasma, serum, or whole blood
in vitro. Resists inactivation by proteolytic enzymes.
Derivative Type: Tartrate
CAS Registry Number: 54974-54-8
Trademarks: Irtonin (Takeda); Xantium (Cyanamid)
Line Formula: C16H22N6O4.xC4H6O6
Therap-Cat: Prohormone.
Keywords: Thyrotropic Hormone.