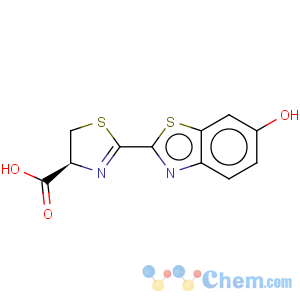
Title: Firefly Luciferin
CAS Registry Number: 2591-17-5
CAS Name: (4
S)-4,5-Dihydro-2-(6-hydroxy-2-benzothiazolyl)-4-thiazolecarboxylic acid
Synonyms: 2-(6-hydroxybenzothiazol-2-yl)-2-thiazoline-4-carboxylic acid; D-(-)-luciferin
Molecular Formula: C11H8N2O3S2
Molecular Weight: 280.32
Percent Composition: C 47.13%, H 2.88%, N 9.99%, O 17.12%, S 22.88%
Literature References: Light emission in the American firefly,
Photinus pyralis, has been shown to involve the interaction of magnesium ion, oxygen, ATP, the enzyme luciferase, and the oxidizable substrate luciferin. Isoln from fireflies (yield from 15,000 active fireflies about 9 mg): Bitler, McElroy,
Arch. Biochem. Biophys. 72, 358 (1957); from Japanese firefly
(Luciola cruciata): Kishi
et al., Tetrahedron Lett. 1968, 2847. Structure and synthesis: E. H. White
et al., J. Am. Chem. Soc. 83, 2402 (1961);
85, 337 (1963);
J. Org. Chem. 30, 2344 (1965); S. Seto
et al., Bull. Chem. Soc. Jpn. 36, 331 (1963). Configuration: G. E. Blank
et al., Biochem. Biophys. Res. Commun. 42, 583 (1971). Measurement of ATP: D. M. Karl, O. Holm-Hansen,
Anal. Biochem. 75, 100 (1976).
Review: L. J. Bowie,
Methods Enzymol. 57, 15 (1978).
Properties: Pale yellow needles from methanol, dec 189.5-190°. Recrystallizes with difficulty and sublimes with decomposition and decarboxylation. [a]D22 -36° (c = 1.2 in DMF). uv max (H2O): 268, 327 nm (log e 3.88, 4.27). Slightly sol in water at pH 6.5. Sol in alkaline aq solns, methanol, acetone, ethyl acetate, DMF. Aqueous solns are sensitive to extremes in pH, esp. in presence of light and oxygen. Racemization occurs rapidly in some solvents. Should be stored for extended periods dry, under nitrogen atmosphere in light-tight containers.
Optical Rotation: [a]D22 -36° (c = 1.2 in DMF)
Absorption maximum: uv max (H2O): 268, 327 nm (log e 3.88, 4.27)
Use: In the assay of ATP.