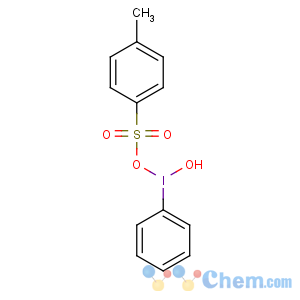
Title: Koser's Reagent
CAS Registry Number: 27126-76-7
CAS Name: Hydroxy(4-methylbenzenesulfonato-k
O)phenyliodine
Synonyms: [hydroxy(tosyloxy)iodo]benzene; HTIB; phenyliodoso hydroxide tosylate; phenylhydroxytosyloxyiodine
Molecular Formula: C13H13IO4S
Molecular Weight: 392.21
Percent Composition: C 39.81%, H 3.34%, I 32.36%, O 16.32%, S 8.18%
Literature References: Unsymmetrical aryl l3-iodane. Prepn: O. Y. Neiland, B. Y. Karele,
J. Org. Chem. USSR 6, 889 (1970). Solid state structure determn by single-crystal x-ray analysis: G. F. Koser
et al., J. Org. Chem. 41, 3609 (1976). Review of use in organic synthesis: R. M. Moriarty
et al., Synlett 1990, 365-383; of chemistry: G. F. Koser,
Aldrichim. Acta 34, 89-102 (2001).
Properties: Stable, nonhygroscopic crystalline solid. Colorless fine needles from dichloroethane, mp 140-142° (Neiland), also reported as colorless prisms from diethyl ether + methanol, mp 136-138.5° (Koser
et al.). Sparingly sol in acetonitrile and dichloromethane at room temp. Readily dissolves in acetonitrile near the reflux temp. to give a yellow soln. Sol in dimethylformamide, alcohols and water with the formation of a yellow color. Sol in chloroform and acetic acid to give a colorless soln. Soly in water at 22°: ca. 1 g/42 mL.
Melting point: mp 140-142° (Neiland), also reported as colorless prisms from diethyl ether + methanol; mp 136-138.5° (Koser
et al.)
Use: Versatile synthetic reagent in phenyliodination and/or tosylation of a range of organic substrates; in oxidative transformations including oxidative rearrangements.