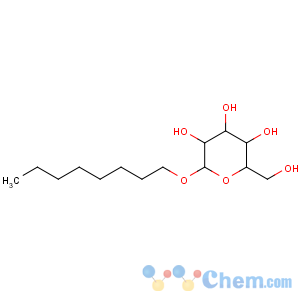
Title: n-Octyl-b-D-glucoside
CAS Registry Number: 29836-26-8
CAS Name: Octyl-b-D-glucopyranoside
Synonyms: n-octylglucoside; OG
Molecular Formula: C14H28O6
Molecular Weight: 292.37
Percent Composition: C 57.51%, H 9.65%, O 32.83%
Literature References: Nonionic detergent primarily used for solubilizing membrane-bound proteins. Prepn: C. R. Noller, C. W. Rockwell,
J. Am. Chem. Soc. 60, 2076 (1938). Partition behavior between water and membrane phases: M. Ueno,
Biochemistry 28, 5631 (1989); thermodynamics and structural impact: M. R. Wenk
et al., Biophys. J. 72, 1719 (1997). Solubilzation and reconstitution of liposomes: O. López
et al., J. Phys. Chem. B 105, 9879 (2001). Solubilization of lipid vesicles: A. Meister, A. Blume,
Phys. Chem. Chem. Phys. 6, 1551 (2004). Micelle formation: A. Walter
et al., Biochim. Biophys. Acta 1508, 20 (2000); in mixed systems: A. Lainez
et al., Langmuir 20, 5745 (2004).
Properties: White solid, mp 65-99°. [a]D25 -30.3° (methanol). Critical micelle concentration: 20-25 m
M.
Melting point: mp 65-99°
Optical Rotation: [a]D25 -30.3° (methanol)
Use: Detergent and surfactant for biological systems.