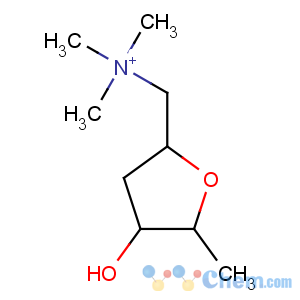
Title: Muscarine
CAS Registry Number: 300-54-9
CAS Name: [2
S-(2a,4b,5a)]-Tetrahydro-4-hydroxy-
N,N,N,5-tetramethyl-2-furanmethanaminium
Molecular Formula: [C9H20NO2]+
Literature References: Alkaloid from the red variety of
Amanita muscaria (L.) Pers.,
Agaricaceae, the fly fungus, a poisonous mushroom. Also found in some other fungi:
Inocybe patouillardi; I. fastigiata, I. umbrina; I. rimosa. Isoln procedure for the naturally occurring L-(+)-form: Kuehl
et al., J. Am. Chem. Soc. 77, 6663 (1955); Eugster,
Helv. Chim. Acta 39, 1002 (1956). Structure and synthesis of racemate: K?gl
et al., Rec. Trav. Chim. 76, 109 (1957); K?gl
et al., Experientia 13, 137, 138 (1957); Cox
et al., Helv. Chim. Acta 41, 229 (1958). Alternate syntheses:
GB 828395 (1960 to Hoffmann-La Roche); Matsumoto
et al., Tetrahedron 25, 5889 (1969); W. C. Still, J. A. Schneider,
J. Org. Chem. 45, 3375 (1980). Synthesis of muscarine: Whiting
et al., Can. J. Chem. 50, 3322 (1972); A. M. Mubarak, D. M. Brown,
Tetrahedron Lett. 21, 2453 (1980);
eidem, J. Chem. Soc. Perkin Trans. 1 1982, 809; S. Pochet, T. Huynhdinh,
J. Org. Chem. 47, 193 (1982). Chemistry and pharmacology: Waser,
Pharmacol. Rev. 13, 465-515 (1961). Toxicity study: P. J. Fraser,
Br. J. Pharmacol. 12, 47 (1957).
Reviews: C. H. Eugster,
Adv. Org. Chem. 2, 427-455 (1960); S. Wilkinson,
Q. Rev. Chem. Soc. 15, 153-171 (1961). Configurational relationship in the muscarine series: Bollinger, Eugster,
Helv. Chim. Acta 54, 2704 (1971).
Derivative Type: Chloride
Molecular Formula: C9H20ClNO2
Molecular Weight: 209.71
Percent Composition: C 51.55%, H 9.61%, Cl 16.91%, N 6.68%, O 15.26%
Properties: Stout prisms from ethanol + acetone, mp 180-181°. Extremely hygroscopic. [a]D25 +8.1° (c = 3.5 in ethanol). Very sol in water, ethanol. Slightly sol in chloroform, ether, acetone. Aq solns are stable. LD50 i.v. in mice: 0.23 mg/kg (Fraser).
Melting point: mp 180-181°
Optical Rotation: [a]D25 +8.1° (c = 3.5 in ethanol)
Toxicity data: LD50 i.v. in mice: 0.23 mg/kg (Fraser)
CAUTION: Potential symptoms of toxicity following ingestion are profuse sweating, increased salivation, visual disturbances, nausea, vomiting, abdominal colic, diarrhea, headache, and bronchospasm. Very high doses may produce lacrimation, incontinence, bradycardia, hypotension and shock.
See Clinical Toxicology of Commercial Products, R. E. Gosselin
et al., Eds. (Williams & Wilkins, Baltimore, 5th ed., 1984) Section II, p 247.
Therap-Cat: Cholinergic.
Keywords: Cholinergic.