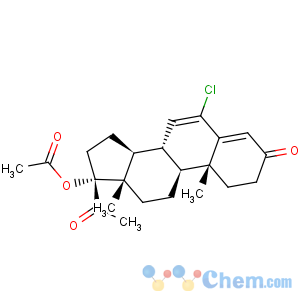
Title: Chlormadinone Acetate
CAS Registry Number: 302-22-7
CAS Name: 17-(Acetyloxy)-6-chloropregna-4,6-diene-3,20-dione
Synonyms: 6-chloro-17-hydroxypregna-4,6-diene-3,20-dione acetate; 6-chloro-6-dehydro-17a-hydroxyprogesterone acetate; 6-chloro-6-dehydro-17a-acetoxyprogesterone; 17a-acetoxy-6-chloro-6,7-dehydroprogesterone
Trademarks: Chronosyn (Veterinaria); Cyclonorm (Streuli); Fertiletten (Chassot); Gestafortin (Merck KGaA); Lormin (Lilly); Luteran (Cassenne); Matrol; Normenon (Syntex); Menstridyl; Prostal (Teikoku Zoki); Traslan (Gramon)
Molecular Formula: C23H29ClO4
Molecular Weight: 404.93
Percent Composition: C 68.22%, H 7.22%, Cl 8.76%, O 15.80%
Literature References: Orally active progestogen with antiandrogenic activity; has been used in combinations as an oral contraceptive. Prepn: Brückner,
DE 1075114 (1960 to E. Merck, AG); Brückner
et al., Ber. 94, 1225 (1961); Sciaky,
Gazz. Chim. Ital. 91, 545 (1961);
GB 932153; H. J. Ringold, A. Bowers,
US 3485852 (1963, 1969 both to Syntex). Endocrinological activities: D. M. Brennan, R. J. Kraay,
Acta Endocrinol. 44, 367 (1963). Metabolism: S. Honma
et al., Chem. Pharm. Bull. 25, 2019 (1977). Clinical evaluation in prostatic carcinoma: R. Nishimura, K. Shida,
Prostate, Suppl. 1, 27 (1981); in benign prostatic hypertrophy: T. Usui
et al., Acta Urol. Jpn. 27, 327 (1981),
B.A. 73, 27225 (1982). Review of carcinogenicity studies:
IARC Monographs 21, 365-375 (1979).
Properties: Crystals from methanol or ether, mp 212-214°. [a]D +6° (c = 1 in CHCl3). uv max: 283.5, 286 nm (e 23400, 22100).
Melting point: mp 212-214°
Optical Rotation: [a]D +6° (c = 1 in CHCl3)
Absorption maximum: uv max: 283.5, 286 nm (e 23400, 22100)
Derivative Type: Mixture with ethinyl estradiol
CAS Registry Number: 37301-55-6
Trademarks: Amenyl; Lutestral (Cassenne); Menova (Merck KGaA)
Derivative Type: Mixture with mestranol
CAS Registry Number: 8065-91-6
Trademarks: C-Quens; Gestamestrol (Hermal); Sequens
Therap-Cat: Progestogen; antineoplastic (hormonal).
Therap-Cat-Vet: Progestogen; estrus regulator.
Keywords: Antineoplastic (Hormonal); Progestogens; Progestogen.