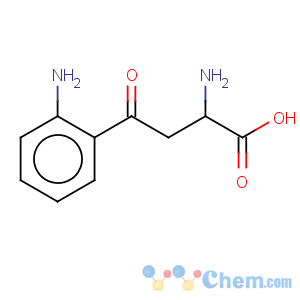
Title: Kynurenine
CAS Registry Number: 343-65-7
CAS Name: a,2-Diamino-g-oxobenzenebutanoic acid
Synonyms: 3-anthraniloylalanine
Molecular Formula: C10H12N2O3
Molecular Weight: 208.21
Percent Composition: C 57.69%, H 5.81%, N 13.45%, O 23.05%
Literature References: An amino acid produced in the body from tryptophan. Isoln from urine of rabbits that had been fed tryptophan: Matsuoka, Yoshimatsu,
Z. Physiol. Chem. 143, 206 (1925); Butenandt
et al., ibid. 279, 27 (1943); Heidelberger
et al., J. Biol. Chem. 179, 143 (1949). Structure and synthesis: Butenandt
et al., loc. cit. Laboratory prepn by oxidation of L-tryptophan with a
Pseudomonas sp.: Hayaishi, Meister,
Biochem. Prep. 3, 108 (1953). From acetyltryptophan: Warnell, Berg,
J. Am. Chem. Soc. 76, 1708 (1954); Auerbach, Knox,
Methods Enzymol. 3, 620 (1957).
Derivative Type: L-Kynurenine hydrate
Molecular Formula: 3C10H12N2O3.H2O
Molecular Weight: 642.66
Percent Composition: C 56.07%, H 5.96%, N 13.08%, O 24.90%
Properties: Leaflets from water, dec 180-190°. [a]D20 -29° (c = 0.4). Slightly sol in water (more sol than the DL-form). Forms a molecular compound with sucrose, C22H34N2O14.H2O, rosettes; dec 145-153°, [a]D21 +14.5° (c = 0.7). Soluble in water.
Optical Rotation: [a]D20 -29° (c = 0.4); [a]D21 +14.5° (c = 0.7)
Derivative Type: L-Kynurenine sulfate monohydrate
Molecular Formula: C10H12N2O3.H2SO4.H2O
Molecular Weight: 324.31
Percent Composition: C 37.03%, H 4.97%, N 8.64%, O 39.47%, S 9.89%
Properties: Needles from water + alcohol. Darkens at 165°, dec 195°. [a]D20 +7.3° (c = 1). uv max (pH 7.0): 230, 257, 360 nm (e 18,900, 7500, 4500). Soluble in water, slightly in alcohol. Kynurenine sulfate requires 3 molecules alkali for neutralization in alcoholic soln.
Optical Rotation: [a]D20 +7.3° (c = 1)
Absorption maximum: uv max (pH 7.0): 230, 257, 360 nm (e 18,900, 7500, 4500)
Derivative Type: L-Kynurenine diacetate
Molecular Formula: C14H16N2O5
Molecular Weight: 292.29
Percent Composition: C 57.53%, H 5.52%, N 9.58%, O 27.37%
Properties: Obtained by acetylating L-kynurenine with ketene. Needles, mp 198°.
Melting point: mp 198°
Derivative Type: Anhydro-L-kynurenine monoacetate
Molecular Formula: C12H12N2O3
Molecular Weight: 232.24
Percent Composition: C 62.06%, H 5.21%, N 12.06%, O 20.67%
Properties: Obtained by acetylating L-kynurenine with acetic anhydride in pyridine. Needles from alc, darkens at 215°, dec 237°.
Derivative Type: DL-Kynurenine sulfate
Molecular Formula: C10H12N2O3.H2SO4
Molecular Weight: 306.29
Percent Composition: C 39.21%, H 4.61%, N 9.15%, O 36.57%, S 10.47%
Properties: Crystals from water + alcohol. Darkens at 166°, dec 194°. Soluble in water. Slightly sol in alcohol.
Use: In biochemical investigations.