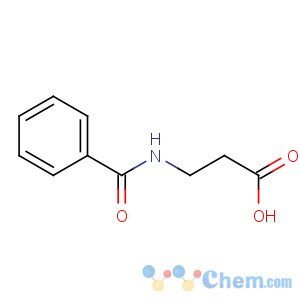
Title: Betamipron
CAS Registry Number: 3440-28-6
CAS Name: N-Benzoyl-b-alanine
Synonyms: 3-(benzoylamino)propionic acid; b-benzamidopropionic acid
Trademarks: CS-443
Molecular Formula: C10H11NO3
Molecular Weight: 193.20
Percent Composition: C 62.17%, H 5.74%, N 7.25%, O 24.84%
Literature References: Acyl amino acid that inhibits active transport of carbapenem antibiotics in the renal cortex.
See also panipenem. Prepn: F. H. Holm,
Arch. Pharm. 242, 590 (1904); C. C. Barker,
J. Chem. Soc. 1954, 317; J. Barluenga
et al., Tetrahedron 45, 2183 (1989). Use as renal protectant: T. Shiokari
et al., EP 226304;
eidem, US 4757066 (1987, 1988 both to Sankyo). Crystal structure: N. Feeder, W. Jones,
Acta Crystallogr. C50, 813 (1994). Nephroprotective effect: Y. Hirouchi
et al., Jpn. J. Pharmacol. 63, 487 (1993). Mode of action:
eidem, ibid. 66, 1 (1994). Pharmacokinetics: A. Kurihara
et al., Antimicrob. Agents Chemother. 36, 1810 (1992).
Properties: Colorless prisms from hot water, mp 120° (Holm); also reported as crystals from water, mp 133° (Barker). Readily sol in warm water, chloroform. Very easily sol in alcohol, ether, acetone. LD50 in rats (mg/kg): >3000 i.v. (Hirouchi, 1994).
Melting point: mp 120° (Holm); mp 133° (Barker)
Toxicity data: LD50 in rats (mg/kg): >3000 i.v. (Hirouchi, 1994)
Therap-Cat: Antibacterial adjunct (renal protectant).
Keywords: Antibacterial Adjuncts; Renal Protectant.