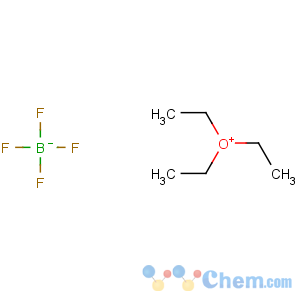
Title: Meerwein's Reagent
CAS Registry Number: 368-39-8
CAS Name: Triethyloxonium tetrafluoroborate (1-)
Synonyms: triethyloxonium fluoborate
Molecular Formula: C6H15BF4O
Molecular Weight: 189.99
Percent Composition: C 37.93%, H 7.96%, B 5.69%, F 40.00%, O 8.42%
Literature References: Powerful ethylating agent; converts alcohols to ethyl ethers at neutral pH. Prepn: H. Meerwein
et al., J. Prakt. Chem. 147, 257 (1937);
idem et al., ibid. 154, 83 (1939); H. Meerwein,
Org. Synth. 46, 113 (1966). Synthetic applications: N. Kornblum, G. P. Coffey,
J. Org. Chem. 31, 3449 (1966); R. Kreher,
Angew. Chem. Int. Ed. 12, 1022 (1973); D. G. McMinn,
Synthesis 1976, 824; D. Crich, H. Dyker,
Tetrahedron Lett. 30, 475 (1989); Y. Yamamoto
et al., Synthesis 1995, 571; A. J. Kiessling, C. K. McClure,
Synth. Commun. 27, 923 (1997).
Brief review: S. Pichlmair,
Synlett 2004, 195-196.
Properties: Colorless solid, mp 91-92° (dec).
Hygroscopic. Store at 0-5° in dichloromethane or diethyl ether.
Melting point: mp 91-92° (dec)
Use: Alkylating agent for nucleophilic functional groups in organic synthesis.