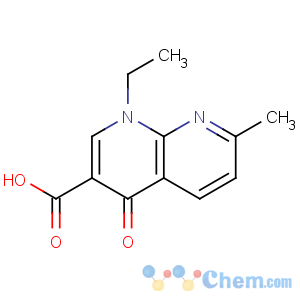
Title: Nalidixic Acid
CAS Registry Number: 389-08-2
CAS Name: 1-Ethyl-1,4-dihydro-7-methyl-4-oxo-1,8-naphthyridine-3-carboxylic acid
Synonyms: 3-carboxy-1-ethyl-7-methyl-1,8-naphthyridin-4-one; 1-ethyl-7-methyl-1,8-naphthyridin-4-one-3-carboxylic acid
Manufacturers' Codes: Win-18320
Trademarks: Betaxina (Terap. M. R.); Eucistin (San Carlo); Innoxalon (Sanko); Nalidicron (Toyo Shinyaku); Nalitucsan (Hishiyama); Narigix (Taiyo); NegGram (Sanofi Winthrop); Negram (Sanofi Winthrop); Nevigramon (Chinoin); Nicelate (Toyo Jozo); Nogram (Sanofi Winthrop); Poleon (Sumitomo); Specifin (Bergamon); Uriben (Rosemont); Uriclar (Crosara); Uralgin (Ceccarelli); Urodixin (Italchimici); Uroman (Toho Kogyo); Uroneg (Ibirn); Uropan (Tiber); Wintomylon (Daiichi)
Molecular Formula: C12H12N2O3
Molecular Weight: 232.24
Percent Composition: C 62.06%, H 5.21%, N 12.06%, O 20.67%
Literature References: Prepn: G. Y. Lesher
et al., J. Med. Pharm. Chem. 5, 1063 (1962); G. Y. Lesher, M. D. Gruett,
BE 612258;
eidem, US 3590036 (1962, 1971 both to Sterling Drug). Mechanism of action studies: G. J. Bourguignon
et al., Antimicrob. Agents Chemother. 4, 479 (1973); W. A. Goss, T. M. Cook,
Antibiotics vol. 3, J. W. Corcoran, F. E. Hahn, Eds. (Springer-Verlag, New York, 1975) pp 174-196; A. M. Pedrini,
ibid. vol. 5 (pt. 1), F. E. Hahn, Ed. (1979) pp 154-175; H. T. Wright
et al., Science 213, 455 (1981). Comprehensive description: P. E. Grubb,
Anal. Profiles Drug Subs. 8, 371-397 (1979).
Properties: Pale buff, crystalline powder, mp 229-230°. Soly at 23° (mg/ml): chloroform 35; toluene 1.6; methanol 1.3; ethanol 0.9; water 0.1; ether 0.1. LD50 in mice (mg/kg): 3300 orally; 500 s.c.; 176 i.v. (Lesher, 1962).
Melting point: mp 229-230°
Toxicity data: LD50 in mice (mg/kg): 3300 orally; 500 s.c.; 176 i.v. (Lesher, 1962)
Therap-Cat: Antibacterial.
Therap-Cat-Vet: Antibacterial.
Keywords: Antibacterial (Synthetic); Quinolones and Analogs.