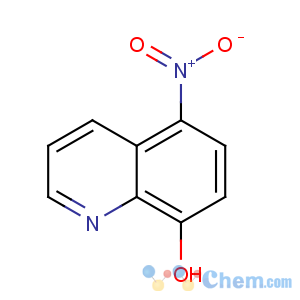
Title: Nitroxoline
CAS Registry Number: 4008-48-4
CAS Name: 5-Nitro-8-quinolinol
Synonyms: 5-nitro-8-hydroxyquinoline
Trademarks: Enterocol; Nibiol; Noxibiol; Uritrol; Urocoli
Molecular Formula: C9H6N2O3
Molecular Weight: 190.16
Percent Composition: C 56.84%, H 3.18%, N 14.73%, O 25.24%
Literature References: Prepn: Kostanecki,
Ber. 24, 154 (1891); Petrow, Sturgeon,
J. Chem. Soc. 1954, 570; Pratt, Duke,
J. Am. Chem. Soc. 82, 1155 (1960).
In vitro antibacterial and antifungal activity: A. Desvignes, P. Leguen,
Ann. Pharm. Fr. 21, 803 (1963); M. Medic-Saric
et al., Chemotherapy 26, 263 (1980). Toxicological study: O. Angelova
et al., Adv. Antimicrob. Antineoplast. Chemother., Proc. 7th Int. Congr. Chemother. 1, 507 (1972). Clinical pharmacokinetics: A. Mrhar
et al., Int. J. Clin. Pharmacol. Biopharm. 17, 476 (1979). HPLC determn in plasma and urine: R. H. A. Sorel
et al., J. Chromatogr. 222, 241 (1981). Clinical evaluation in urinary tract infections: M. R. Jacobs
et al., S. Afr. Med. J. 54, 959 (1978); B. Cancet, A. Amgar,
Pathol. Biol. 35, 879 (1987).
Properties: Yellow needles from alcohol or acetic acid, mp 179.5-181.5°. Freely sol in alkali and hot HCl; sparingly sol in alcohol, ether.
Melting point: mp 179.5-181.5°
Derivative Type: Hydrochloride
Molecular Formula: C9H7ClN2O3
Molecular Weight: 226.62
Percent Composition: C 47.70%, H 3.11%, Cl 15.64%, N 12.36%, O 21.18%
Properties: Yellow needles from alcohol, mp 258°.
Melting point: mp 258°
Therap-Cat: Antibacterial.
Keywords: Antibacterial (Synthetic).