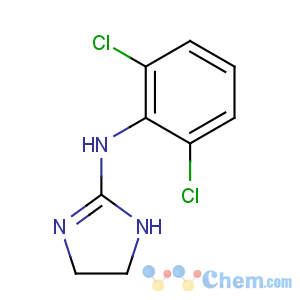
Title: Clonidine
CAS Registry Number: 4205-90-7
CAS Name: N-(2,6-Dichlorophenyl)-4,5-dihydro-1
H-imidazol-2-amine
Synonyms: 2-(2,6-dichloroanilino)-2-imidazoline; 2,6-dichloro-
N-2-imidazolidinylidenebenzenamine; 2-(2,6-dichloroanilino)-1,3-diazacyclopentene-(2); 2-[(2,6-dichlorophenyl)imino]-2-imidazoline
Molecular Formula: C9H9Cl2N3
Molecular Weight: 230.09
Percent Composition: C 46.98%, H 3.94%, Cl 30.82%, N 18.26%
Literature References: a2-Adrenergic agonist. Prepn: Zeile
et al., US 3202660 (1965 to Boehringer, Ing.). Use in shaving soap formulations:
eidem, US 3190802 (1965 to Boehringer, Ing.). Pharmacology: Bolme, Fuxe,
Eur. J. Pharmacol. 13, 168 (1971). Revised structure: L. M. Jackman, T. Jen,
J. Am. Chem. Soc. 97, 2811 (1975). GC determn in plasma: P. O. Edlund,
J. Chromatogr. 187, 161 (1980). Preliminary studies on potential antidepressant activity: D. C. Jimerson
et al., Biol. Psychiatry 15, 45 (1980); J. B. Malick,
Prog. Clin. Biol. Res. 71, 165 (1981). Exptl use in drug rehabilitation: M. S. Gold, A. C. Pottash,
Ann. N.Y. Acad. Sci. 362, 191-202 (1981). Activity as a-adrenoceptor agonist: A. G. Roach
et al., J. Pharmacol. Exp. Ther. 227, 421 (1983). Symposium on cardiovascular and psychotropic pharmacology and clinical experience:
Central Blood Pressure Regulation: The Role of a
2-Receptor Stimulation, K. Hayduk, K. B. Bock, Eds. (Steinkopff Verlag, Darmstadt, 1983) 284 pp. Effects in acute smoking withdrawal syndrome: A. H. Glassman
et al., Science 226, 864 (1984); in alcoholism withdrawal: Z. Jraidi
et al., Therapie 42, 21 (1987). Clinical trial in Tourette's syndrome: J. F. Leckman
et al., Neurology 35, 343 (1985); in cigarette smoking cessation: R. Davison
et al., Clin. Pharmacol. Ther. 44, 265 (1988). Clinical trial for intractable cancer pain: J. C. Eisenach
et al., Pain 61, 391 (1995).
Reviews: A. Walland in
Pharmacological and Biochemical Properties of Drug Substances vol. 1, M. E. Goldberg, Ed. (Am. Pharm. Assoc., Washington, D.C., 1977) pp 67-107; H. Schmitt,
Handb. Exp. Pharmacol. 39, 299-396 (1977); M. C. Houston,
Prog. Cardiovasc. Dis. 23, 337-350 (1981). Comprehensive description: M. A. Abounassif
et al., Anal. Profiles Drug Subs. Excip. 21, 109-147 (1992).
Properties: Crystals, mp 130°.
Melting point: mp 130°
Derivative Type: Hydrochloride
CAS Registry Number: 4205-91-8
Manufacturers' Codes: ST-155
Trademarks: Catapres (Boehringer, Ing.); Catapresan (Boehringer, Ing.); Clonistada (Stadapharm); Dixarit (Boehringer, Ing.); Duraclon (Roxane); Isoglaucon (Boehringer, Ing.); Tenso-Timelets
Molecular Formula: C9H9Cl2N3.HCl
Molecular Weight: 266.55
Percent Composition: C 40.55%, H 3.78%, Cl 39.90%, N 15.76%
Properties: Crystals, mp 305°. Sol in abs. ethanol; slightly sol in chloroform. Practically insol in ether. One gram is sol in 6 mls water (60°C); about 13 mls water (20°C); about 5.8 mls methanol; about 25 mls ethanol; about 5000 mls chloroform. uv max (water): 213, 271, 302 nm (e 8290.327, 713.074, 339.876). LD50 in mice, rats (mg/kg): 328, 270 orally; 18, 29 i.v. (Walland).
Melting point: mp 305°
Absorption maximum: uv max (water): 213, 271, 302 nm (e 8290.327, 713.074, 339.876)
Toxicity data: LD50 in mice, rats (mg/kg): 328, 270 orally; 18, 29 i.v. (Walland)
Use: In shaving soaps.
Therap-Cat: Antihypertensive; analgesic for neuropathic pain.
Keywords: a-Adrenergic Agonist; Antidyskinetic; Antihypertensive; Imidazole Derivatives.