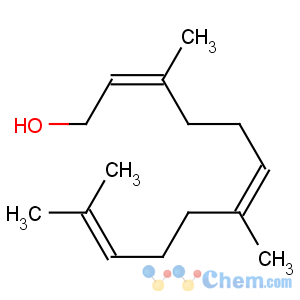
Title: Farnesol
CAS Registry Number: 4602-84-0
CAS Name: 3,7,11-Trimethyl-2,6,10-dodecatrien-1-ol
Molecular Formula: C15H26O
Molecular Weight: 222.37
Percent Composition: C 81.02%, H 11.79%, O 7.19%
Literature References: Found in oils of citronella, neroli, cyclamen, lemon grass, tuberose, rose, musk, balsam Peru, and tolu. Isoln: Elge,
Chem. Ztg. 34, 857 (1910);
37, 1422 (1913); Kerschbaum,
Ber. 46, 1732 (1913); Naves,
Helv. Chim. Acta 32, 1798, 2181 (1949); LaFace,
ibid. 33, 249 (1950). Synthesis: Ruzicka,
ibid. 6, 492 (1923); Ruzicka, Firmenich,
ibid. 22, 392 (1939); Nazarov
et al., Zh. Obshch. Khim. 28, 1444 (1958); Shvarts, Petrov,
ibid. 30, 3598 (1960); Popjak
et al., J. Biol. Chem. 237, 56 (1962). Four possible stereoisomers. Stereochemistry: Bates
et al., Chem. Ind. (London) 1961, 1907;
J. Org. Chem. 28, 1086 (1963).
trans-trans-Farnesol is the only stereoisomer present in many essential oils but occurs mixed with
cis-trans-farnesol in petitgrain oil and several other oils: Naves,
Compt. Rend. 251, 900 (1960). Stereospecific synthesis of
trans-trans-farnesol: Corey
et al., J. Am. Chem. Soc. 92, 6637 (1970).
Derivative Type: trans-trans-Farnesol
Properties: Liquid. bp0.35 111°.
nD25 1.4872. uv max: 192-196 nm (e 28,500).
Boiling point: bp0.35 111°
Index of refraction: nD25 1.4872
Absorption maximum: uv max: 192-196 nm (e 28,500)
Derivative Type: Commercial farnesol
Properties: bp0.2 110-113°. d420 0.8871.
nD20 1.4870.
Boiling point: bp0.2 110-113°
Index of refraction: nD20 1.4870
Density: d420 0.8871
Use: In perfumery, to emphasize the odor of sweet floral perfumes, such as lilac and cyclamen.