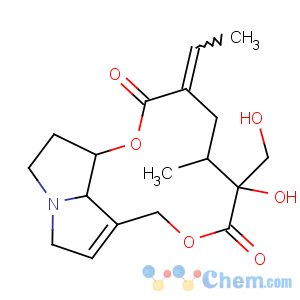
Title: Retrorsine
CAS Registry Number: 480-54-6
CAS Name: 12,18-Dihydroxysenecionan-11,16-dione
Synonyms: b-longilobine
Molecular Formula: C18H25NO6
Molecular Weight: 351.39
Percent Composition: C 61.52%, H 7.17%, N 3.99%, O 27.32%
Literature References: Hepatotoxic pyrrolizidine alkaloid; common constituent of
Senecio species. Isoln from
Senecio retrorsus DC,
Compositae: R. H. F. Manske,
Can. J. Chem. 5, 651 (1931); G. Barger
et al., J. Chem. Soc. 1935, 11; from
Crotalaria usaramoensis E. G. Baker,
Leguminosae: C. C. J. Culvenor, L. W. Smith,
Aust. J. Chem. 19, 2127 (1966). Structure: S. M. H. Christie
et al., J. Chem. Soc. 1949, 1700; E. C. Leisegang, F. L. Warren,
ibid. 1950, 702. Identity with b-longilobine: F. L. Warren
et al., J. Am. Chem. Soc. 72, 1421 (1950). Review and evaluation of toxicity and carcinogenicity studies:
IARC Monographs 10, 303-312, 333-342 (1976). Comprehensive reviews of pyrrolizidine alkaloids: L. B. Bull
et al., The Pyrrolizidine Alkaloids (North Holland, Amsterdam, 1968) 293 pp; F. L. Warren in
The Alkaloids vol. 12, R. H. F. Manske, Ed. (Academic Press, New York, 1970) pp 245-331.
Properties: Crystals from ethyl acetate, mp 212° (Barger
et al.); 216-216.5° (Bull
et al.). [a]D18 -17.6° (c = 1.99 in ethanol). uv max (water): 217 nm (log e 3.85). Readily sol in alcohol, chloroform; slightly sol in water, acetone, ethyl acetate; practically insol in ether.
Melting point: mp 212° (Barger
et al.); 216-216.5° (Bull
et al.)
Optical Rotation: [a]D18 -17.6° (c = 1.99 in ethanol)
Absorption maximum: uv max (water): 217 nm (log e 3.85)
Derivative Type: N-Oxide
Synonyms: Isatidine; 12,18-dihydroxysenecionan-11,16-dione-4-oxide; retrorsine
N-oxide
Molecular Formula: C18H25NO7
Molecular Weight: 367.39
Percent Composition: C 58.85%, H 6.86%, N 3.81%, O 30.48%
Literature References: Isolated from
Senecio species. Review and evaluation of carcinogenicity and toxicity studies:
IARC Monographs 10, 269-273 (1976).
Properties: Crystals from ethanol, mp 140.5-141.5° (Christie
et al.).
Melting point: mp 140.5-141.5° (Christie
et al.)