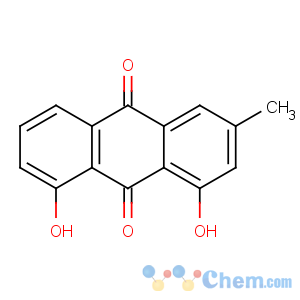
Title: Chrysophanic Acid
CAS Registry Number: 481-74-3
CAS Name: 1,8-Dihydroxy-3-methyl-9,10-anthracenedione
Synonyms: 1,8-dihydroxy-3-methylanthraquinone; 3-methylchrysazin; chrysophanol
Molecular Formula: C15H10O4
Molecular Weight: 254.24
Percent Composition: C 70.86%, H 3.96%, O 25.17%
Literature References: Occurs in the free state and as glucoside in cascara sagrada, senna and various species of
Rumex and
Rheum (rhubarb). Isoln from rhubarb root: Tutin, Clewer,
J. Chem. Soc. 99, 946 (1911); Siesto, Bartoli,
Farmaco Ed. Prat. 12, 517 (1957); Carelli, Giuliano,
ibid. 184; from
Penicillium islandicum Sopp.: Howard, Raistrick,
Biochem. J. 46, 49 (1950); from
Chaetonium affine Corda: Arkley
et al., Croat. Chem. Acta 29, 141 (1957),
C.A. 53, 1287h (1959). Synthesis: Eder, Widmer,
Helv. Chim. Acta 5, 3 (1922);
6, 419 (1923); Ayyangar
et al., J. Sci. Ind. Res. 20B, 493 (1961). Total synthesis: M. E. Jung, J. A. Lowe,
Chem. Commun. 1978, 95.
Properties: Hexagonal or monoclinic crystals from alcohol or benzene, mp 196°. Sublimes. Absorption max: 226, 256, 278, 288, 436 nm (e ′ 10-3 41, 28, 14, 14, 11.8). Practically insol in water. Slightly sol in cold, freely in boiling alc; sol in benzene, chloroform, ether, glacial acetic acid, acetone, solns of alkali hydrides, and in hot solns of alkali carbonates; very slightly sol in petr ether.
Melting point: mp 196°
Absorption maximum: Absorption max: 226, 256, 278, 288, 436 nm (e ′ 10-3 41, 28, 14, 14, 11.8)
Derivative Type: Glucoside
Synonyms: Chrysophanein; chrysophaniin
Molecular Formula: C21H20O9
Molecular Weight: 416.38
Percent Composition: C 60.58%, H 4.84%, O 34.58%
Properties: Fine yellow needles from alc, mp 248-249°. Slightly sol in hot water; sol in pyridine. Practically insol in cold water, chloroform, ether.
Melting point: mp 248-249°