References of 3-Pyrrolidineaceticacid, 2-carboxy-4-(1-methylethenyl)-, (2S,3S,4S)-
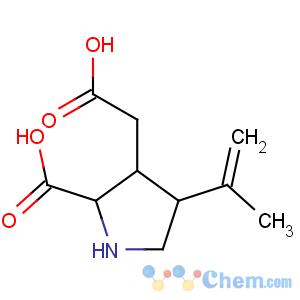
Title: Kainic Acid
CAS Registry Number: 487-79-6
CAS Name: [2
S-(2a,3b,4b)]-2-Carboxy-4-(1-methylethenyl)-3-pyrrolidineacetic acid
Synonyms: 2-carboxy-3-carboxymethyl-4-isopropenylpyrrolidine; digenic acid; a-kainic acid; LS-
xylo-kainic acid
Trademarks: Digenin; Helminal
Molecular Formula: C10H15NO4
Molecular Weight: 213.23
Percent Composition: C 56.33%, H 7.09%, N 6.57%, O 30.01%
Literature References: Anthelmintic principle from the dried red alga
Digenea simplex (Wulf.) Ag.,
Rhodomelaceae: Murakami
et al., J. Pharm. Soc. Jpn. 73, 1026 (1953);
JP 54 4947 (1954),
C.A. 49, 13604i (1955); Katsuya
et al., JP 64 1942 (1964 to New-Japan Pharmaceutical Co). Structure: Watase, Nitta,
Bull. Chem. Soc. Jpn. 30, 889 (1957); Watase
et al., ibid. 31, 714 (1958). Eight theoretical stereoisomers, Nitta
et al., Nature 181, 761 (1958);
GB 795750 (1958 to Takeda). Synthesis: Ueno,
US 2902492; Tatsuoka
et al., US 2954384 (1959, 1960 both to Takeda); W. Oppolzer, H. Andres,
Helv. Chim. Acta 62, 2282 (1979). Excitotoxic amino acid used to identify a specific subset of EAA receptors. Consequently the receptors are known as kainate receptors. Neurotoxic activity: J. V. Nadler,
Life Sci. 24, 289 (1979). Mechanism of neurotoxicity: E. G. McGeer
et al., Adv. Neurol. 23, 577 (1979); J. T. Coyle
et al., ibid. 593; J. W. Ferkany
et al., Nature 298, 757 (1982); J. Garthwaite, G. Garthwaite,
ibid. 305, 138 (1983). Induces epileptogenic lesions: J. V. Nadler,
Neurosci. Res. Program Bull. 19, 369 (1981). Autoradiographic characterization of binding sites: J. T. Greenamyre
et al., J. Pharmacol. Exp. Ther. 233, 254 (1985).
Book: Kainic Acid as a Tool in Neurobiology, E. G. McGeer
et al., Eds. (Raven Press, New York, 1978).
Review: J. T. Coyle,
Ciba Found. Symp. 126, 186-203 (1987).
Properties: Needles, dec 251°. [a]D24 -14.8° (c = 1.01). Intense absorption at 6.05 and 11.2 m. Sol in water. Insol in ethanol. Stable in boiling aq solns.
Optical Rotation: [a]D24 -14.8° (c = 1.01)
Use: Neurobiological tool.
Therap-Cat: Anthelmintic (Nematodes).
Keywords: Anthelmintic (Nematodes).