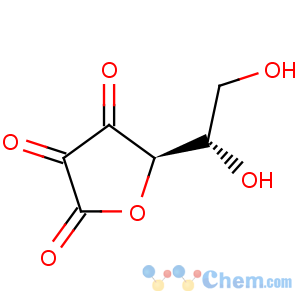
Title: Dehydroascorbic Acid
CAS Registry Number: 490-83-5
CAS Name: L-
threo-2,3-Hexodiulosonic acid g-lactone
Molecular Formula: C6H6O6
Molecular Weight: 174.11
Percent Composition: C 41.39%, H 3.47%, O 55.14%
Literature References: The reversibly oxidized form of ascorbic acid. Prepd by the action of benzoquinone on ascorbic acid: Ohle, Erlbach,
Ber. 67, 555 (1934); Moll, Wieters,
E. Merck's Jahresber. 50, 65 (1936); by the action of iodine: Herbert
et al., J. Chem. Soc. 1933, 1270; Kenyon, Munro,
ibid. 1948, 158; by oxidn with
peri-naphthindan-2,3,4-trione hydrate: Moubasher,
J. Biol. Chem. 176, 533 (1948);
see also Müller-Mulot,
Z. Physiol. Chem. 351, 52 (1970). Isomerization and formn of derivs: Egge,
Tetrahedron Lett. 1969, 801. Structure studies: Teichmann, Ziebarth,
Z. Prakt. Chem. 33, 124 (1966). Toxicity studies: Gaudiano
et al., Boll. Soc. Ital. Biol. Sper. 43, 674 (1967).
Properties: Fine needles, dec 225°. Sol in water at 60°. In soln the two carbonyl groups (in position 2 and 3) assume the hydrated form -C(OH)2-C(OH)2-. Practically neutral reaction. pKa: 3.90. [a]D20 +56°. Aq solns are much less stable than those of ascorbic acid. Detailed stability data: Bogdanski, Bogdanska,
Bull. Acad. Pol. Sci. Cl. 2 3, 41 (1955).
See also Velisek
et al., Collect. Czech. Chem. Commun. 37, 1465 (1972). Undecomposed dehydroascorbic acid in soln is easily converted to ascorbic acid by reduction with sulfurous acid. Has same antiscorbutic activity in humans as ascorbic acid (upon oral ingestion).
pKa: pKa: 3.90
Optical Rotation: [a]D20 +56°