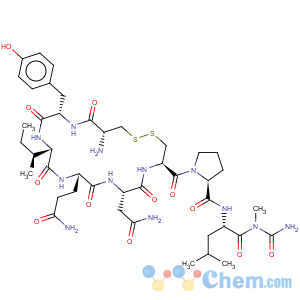
Title: Oxytocin
CAS Registry Number: 50-56-6
Synonyms: Alpha-hypophamine; ocytocin
Trademarks: Intertocine-S (Veterinaria); Perlacton (Chassot); Pitocin (Monarch); Syntocinon (Novartis); Orasthin (Aventis); Partocon (Ferring); Synpitan (Werfft); Uteracon (National)
Molecular Formula: C43H66N12O12S2
Molecular Weight: 1007.19
Percent Composition: C 51.28%, H 6.60%, N 16.69%, O 19.06%, S 6.37%
Literature References: The principal uterus-contracting and lactation-stimulating hormone of the posterior pituitary gland. Isoln: Pierce
et al., J. Biol. Chem. 199, 929 (1952). Structure and synthesis: Tuppy, Michl,
Monatsh. Chem. 84, 1011 (1953); Tuppy,
Biochim. Biophys. Acta 11, 449 (1953); du Vigneaud
et al., J. Am. Chem. Soc. 75, 4879 (1953);
76, 3115 (1954); Bodanszky, du Vigneaud,
ibid. 81, 2504 (1959); Cash
et al., J. Med. Pharm. Chem. 5, 413 (1962); Sakakibara
et al., Bull. Chem. Soc. Jpn. 38, 120 (1965). Solid phase synthesis: Bayer, Hagenmaier,
Tetrahedron Lett. 1968, 2037; Ives,
Can. J. Chem. 46, 2318 (1968). Synthesis of D-oxytocin: Flouret, du Vigneaud,
J. Am. Chem. Soc. 87, 3775 (1965). Description of commercial process: Velluz
et al., US 2938891 and
US 3076797 (1960, 1963, both to Roussel-UCLAF). Radioimmunoassay: T. Chard,
Clin. Biochem. Anal. 5, 209 (1977).
Review: du Vigneaud,
Experientia Suppl. II (14th Intl. Congr. Pure and Appl. Chem.), 9-26 (1955); R. Caldeyro-Barcia, H. Heller,
Proc. Intl. Symp. on Oxytocin (Montevideo 1959) 443 pp; several authors,
Adv. Exp. Med. Biol. 2, 53-104 (1968); C. R. W. Edwards in
Hormones in Blood vol. 2, C. H. Gray, V. James, Ed. (Academic Press, New York, 3rd ed., 1979) pp 401-421. Review of role in parturition: A.-R. Fuchs, F. Fuchs,
Adv. Exp. Med. 1980, 403-428. Comprehensive description: F. Nachtmann
et al., Anal. Profiles Drug Subs. 10, 563-600 (1981). Book:
Oxytocin: Cellular and Molecular Approaches in Medicine and Research, R. Ivell, J. A. Russell, Eds. (Plenum Press, New York, 1995) 673 pp.
Properties: White powder. [a]D22 -26.2° (c = 0.53). Sol in water, 1-butanol, 2-butanol.
Optical Rotation: [a]D22 -26.2° (c = 0.53)
Therap-Cat: Oxytocic.
Therap-Cat-Vet: Stimulates milk let-down, uterine contraction.
Keywords: Oxytocic.