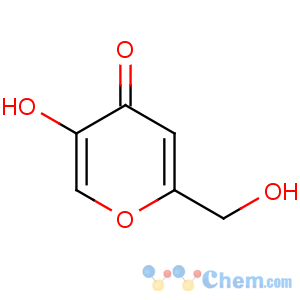
Title: Kojic Acid
CAS Registry Number: 501-30-4
CAS Name: 5-Hydroxy-2-(hydroxymethyl)-4
H-pyran-4-one
Synonyms: 5-hydroxy-2-(hydroxymethyl)-4-pyrone; 2-hydroxymethyl-5-hydroxy-g-pyrone
Molecular Formula: C6H6O4
Molecular Weight: 142.11
Percent Composition: C 50.71%, H 4.26%, O 45.03%
Literature References: Antibiotic substance produced in an aerobic process by a variety of microorganisms from a wide range of carbon sources. Isoln from
Aspergillus oryzae: Saito,
Bot. Mag. Tokyo 21, 249 (1907). Structure: Yabuta,
J. Chem. Soc. 125, 575 (1924); Heyns, Vogelsang,
Ber. 87, 13 (1954). Synthesis: Stacey, Turton,
J. Chem. Soc. 1946, 661; Lichtenthaler, Heidel,
Angew. Chem. Int. Ed. 8, 978 (1968). Industrial prepn:
GB 826244 (1959 to Pfizer),
C.A. 54, 11372e (1960). Mutagenicity study: C. I. Wei
et al., Toxicol. Lett. 59, 213 (1991).
Review: Beélik,
Adv. Carbohydr. Chem. 11, 145-183 (1956); Wilson, "Miscellaneous
Aspergillus Toxins" in
Microbial Toxins vol. VI, A. Ciegler
et al., Eds. (Academic Press, New York, 1971) pp 235-250.
Properties: Prismatic needles from acetone, ethanol + ether or methanol+ ethyl acetate, mp 153-154°. pKa 7.90, 8.03. Freely sol in water, ethanol, acetone; sparingly sol in ether, ethyl acetate, chloroform, pyridine. Absorption spectrum: Stacey, Turton.
Melting point: mp 153-154°
pKa: pKa 7.90, 8.03
Use: Converted to maltol and ethyl maltol, flavor-enhancing additives. Food additive to inhibit tyrosinase.