Title: Meglutol
CAS Registry Number: 503-49-1
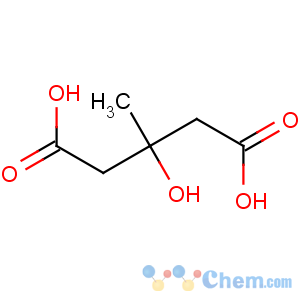
CAS Name: 3-Hydroxy-3-methylpentanedioic acid
Synonyms: 3-hydroxy-3-methylglutaric acid; dicrotalic acid; medroglutaric acid; HMG; HMGA
Manufacturers' Codes: CB-337
Trademarks: Lipoglutaren (Ausonia); Mevalon (Guidotti)
Molecular Formula: C6H10O5
Molecular Weight: 162.14
Percent Composition: C 44.45%, H 6.22%, O 49.34%
Literature References: Hypolipidemic agent that decreases the rate of cholesterol synthesis. Prepn via Reformatsky reaction between ethyl acetoacetate and ethyl bromoacetate: R. Adams, B. L. Van Duuren,
J. Am. Chem. Soc. 75, 2377 (1953). Synthesis via oxidation of diallylmethylcarbinol with ozone and hydrogen peroxide: H. J. Klosterman, F. Smith,
ibid. 76, 1229 (1954); J. L. Rabinowitz,
Biochem. Prep. 6, 25 (1958).
Caution: attempts to increase batch size of the ozonolysis resulted in an explosion,
cf. Chem. Eng. News 51(6), 29 (1973). Improved synthesis: A. Yavrouian
et al., Synthesis 1981, 791. Hypocholesteremic properties: Z. H. Beg, M. Siddiqi,
Experientia 23, 380 (1967); M. Siddiqi, Z. H. Beg,
US 3629449 (1971); Z. H. Beg, P. J. Lupien,
Biochim. Biophys. Acta 260, 439 (1972); A. N. K. Yusufi, M. Siddiqi,
Atherosclerosis 20, 517 (1974); C. D. Padova
et al., Life Sci. 30, 1907 (1982). Biosynthetic studies: L. W. White, H. Rudney,
Biochemistry 9, 2713 (1970); L. Hagenfeldt, K. Hellstrom,
Life Sci. 9, Pt. 2, 991 (1970). Organ distribution: L. L. Savoie, P. J. Lupien,
Can. J. Physiol. Pharmacol. 53, 638 (1975). Effectiveness in familial hypercholesteremia: P. J. Lupien
et al., J. Clin. Pharmacol. 19, 120 (1979). GC study in patients with organic acidurias: K. Tanaka, D. J. Hine,
J. Chromatogr. 239, 301 (1982). Toxicological study: L. L. Savoie, P. J. Lupien,
Arzneim.-Forsch. 25, 1284 (1975).
Properties: Cryst from ether/petr ether, mp 108-109°. Sol in water. Stable when stored dry. LD50 in mice (g/kg): 7.33 orally; 3.23 i.p. (Savoie, Lupien).
Melting point: mp 108-109°
Toxicity data: LD50 in mice (g/kg): 7.33 orally; 3.23 i.p. (Savoie, Lupien)
Therap-Cat: Antilipemic.
Keywords: Antilipemic.