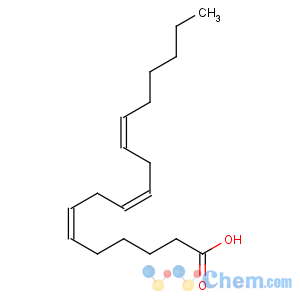
Title: g-Linolenic Acid
CAS Registry Number: 506-26-3
CAS Name: (6
Z,9
Z,12
Z)-6,9,12-Octadecatrienoic acid
Synonyms: cis-6,
cis-9,
cis-12-octadecatrienoic acid; gamolenic acid; GLA
Trademarks: Viacutan (Boehringer, Ing.)
Molecular Formula: C18H30O2
Molecular Weight: 278.43
Percent Composition: C 77.65%, H 10.86%, O 11.49%
Literature References: Polyunsaturated fatty acid produced in the body as the D6-desaturase metabolite of linoleic acid,
q.v. Converted to dihomo-g-linolenic acid, a biosynthetic precursor of monoenoic prostaglandins such as PGE1. Present to varying extents in the fatty acid fraction of evening primrose oil (7-10%), in borage oil (18-26%), in black currant oil (15-20%) and in oils from different fungal sources (6-24%). Isoln from evening primrose oil,
q.v.: A. Heiduschka, K. Luft,
Arch. Pharm. 257, 33 (1919). Proposed structure: Eibner
et al., Chem. Umschau. 34, 312 (1927). Confirmation of structure: J. P. Riley,
J. Chem. Soc. 1949, 2728. Discussion of occurrence, esp. in fungi: R. Shaw,
Biochim. Biophys. Acta 98, 230 (1965). Synthesis: J. M. Osbond
et al., J. Chem. Soc. 1961, 2779; J. M. Osbond,
ibid. 5270. Metabolism studies: J. F. Mead, D. R. Howton,
J. Biol. Chem. 229, 575 (1957); K. J. Stone
et al., Lipids 14, 174 (1979). Effect of source on essential fatty acid and prostanoid metabolite formation: D. K. Jenkins
et al., Med. Sci. Res. 16, 525 (1988).
Derivative Type: Hexabromide deriv
Molecular Formula: C18H30Br6O2
Molecular Weight: 757.85
Percent Composition: C 28.53%, H 3.99%, Br 63.26%, O 4.22%
Properties: Crystals from ethyl methyl ketone, mp 201-202°.
Melting point: mp 201-202°
Use: Nutrient.
Therap-Cat: In treatment of atopic eczema.
Therap-Cat-Vet: Dermatological.
Keywords: Antieczematic.